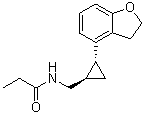
Tasimelteon (formerly BMS-214778; VEC-162; trade name: Hetlioz), a novel circadian regulator, is the first medication approved by both FDA and European Medicines Agency (EMA) for the treatment of Non-24-hour Sleep-Wake Disorder (Non-24), or sleep-wake disorder in blind individuals. Tasimelteon is a strong and selective agonist of the melatonin (MT1 and MT2) receptor that has a 2- to 4-fold higher affinity for the MT2 receptor. Tasimelteon therapy over the long term was risk-free and well-tolerated. Placebo-controlled data in patients with insomnia and those who are not 24 support this.
Physicochemical Properties
| Molecular Formula | C15H19NO2 | |
| Molecular Weight | 245.32 | |
| Exact Mass | 245.141 | |
| Elemental Analysis | C, 73.44; H, 7.81; N, 5.71; O, 13.04 | |
| CAS # | 609799-22-6 | |
| Related CAS # | Tasimelteon-d5; 1962124-51-1 | |
| PubChem CID | 10220503 | |
| Appearance | Solid powder | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 442.6±24.0 °C at 760 mmHg | |
| Melting Point | 78°C(lit.) | |
| Flash Point | 221.4±22.9 °C | |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C | |
| Index of Refraction | 1.564 | |
| LogP | 1.75 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 2 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 18 | |
| Complexity | 318 | |
| Defined Atom Stereocenter Count | 2 | |
| SMILES | O1C([H])([H])C([H])([H])C2C1=C([H])C([H])=C([H])C=2[C@]1([H])C([H])([H])[C@@]1([H])C([H])([H])N([H])C(C([H])([H])C([H])([H])[H])=O |
|
| InChi Key | PTOIAAWZLUQTIO-GXFFZTMASA-N | |
| InChi Code | InChI=1S/C15H19NO2/c1-2-15(17)16-9-10-8-13(10)11-4-3-5-14-12(11)6-7-18-14/h3-5,10,13H,2,6-9H2,1H3,(H,16,17)/t10-,13+/m0/s1 | |
| Chemical Name | N-[[(1R,2R)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl]methyl]propanamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | MT2 receptor ( pKi = 9.8 ); MT1 receptor ( pKi = 9.45 ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Following oral administration of radiolabeled tasimelteon, 80% of total radioactivity was excreted in urine and approximately 4% in feces, resulting in a mean recovery of 84%. Less than 1% of the dose was excreted in urine as the parent compound. The apparent oral volume of distribution of tasimelteon at steady state in young healthy subjects is approximately 56 - 126 L. Metabolism / Metabolites Tasimelteon is extensively metabolized. Metabolism of tasimelteon consists primarily of oxidation at multiple sites and oxidative dealkylation resulting in opening of the dihydrofuran ring followed by further oxidation to give a carboxylic acid. CYP1A2 and CYP3A4 are the major isozymes involved in the metabolism of tasimelteon. Phenolic glucuronidation is the major phase II metabolic route. Biological Half-Life The observed mean elimination half-life for tasimelteon is 1.3 ± 0.4 hours. |
||
| Toxicity/Toxicokinetics |
Hepatotoxicity In several clinical trials, tasimelteon was found to be well tolerated. Serum enzyme elevations occurred in up to 10% of tasimelteon treated patients compared to 5% of placebo controls, but instances of clinically apparent liver injury were not reported. In a combined analysis of 6 trials of tasimelteon given for an average of 1 year, ALT elevations above 3 times the ULN arose in 6.5% of tasimelteon treated subjects, but no elevations were above 10 times ULN, and none were associated with symptoms or jaundice. Most elevations were single values and resolved spontaneously without dose reduction or discontinuation. Tasimelteon has been available for a limited period of time, but has not been linked to instances of clinically apparent liver injury. Tasimelteon is metabolized by the cytochrome P450 system (predominantly CYP 1A2 and CYP3A4), which can result in significant drug-drug interactions, strong inhibitors of the enzymes increasing serum concentrations of tasimelteon and strong inducers decreasing them. Likelihood score: E (unlikely cause of clinically apparent liver injury). Drug Class: Sedatives and Hypnotics Other Drugs in the Subclass, Melatonin and its Analogues: Melatonin, Ramelteon Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of tasmelteon during breastfeeding. Monitor the infant for drowsiness and adequate feeding, especially while nursing a newborn or preterm infant. Until more data become available an alternate drug may be preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding At therapeutic concentrations, tasimelteon is about 90% bound to proteins. |
||
| References |
[1]. Expert Opin Investig Drugs . 2011 Jul;20(7):987-93. [2]. Am J Ther . 2015 Sep-Oct;22(5):355-60. [3]. J Clin Pharmacol. 2015 May; 55(5): 525–533. |
||
| Additional Infomation |
Tasimelteon is a member of the class of 1-benzofurans that is propionamide in which one of the amide hydrogens is replaced by a [(1R,2R)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl]methyl group. A melatonin receptor agonist used for the treatment of non-24-hour sleep-wake disorder. It has a role as a melatonin receptor agonist. It is a monocarboxylic acid amide, a member of 1-benzofurans and a member of cyclopropanes. It is functionally related to a propionamide. Tasimelteon is a selective dual melatonin receptor agonist indicated for the treatment of Non-24-Hour Sleep-Wake Disorder (N24HSWD). Occurring commonly in blind individuals without light perception, this condition is often characterized by periods of night-time insomnia and day-time sleepiness. In blind individuals, a lack of light stimulation causes an extension of the 24-hour circadian cycle and can lead to progressively delayed sleep onset. By activating melatonin receptors MT1 and MT2 in the suprachiasmatic nucleus of the brain, tasimelteon has been shown to improve sleep by resynchronizing the circadian rhythm through its "non-photic" mechanism. Tasimelteon is currently the only drug available for the treatment of N24HSWD and was granted orphan drug status by the FDA in 2010. Tasimelteon is a Melatonin Receptor Agonist. The mechanism of action of tasimelteon is as a Melatonin Receptor Agonist. Tasimelteon is a melatonin receptor agonist that is used for the treatment of non-24 hour sleep-wake disorder in blind individuals. Tasimelteon therapy is associated with a low rate of serum enzyme elevations, but has not been implicated in cases of clinically apparent liver injury. Drug Indication Tasimelteon oral capsules are indicated for the treatment of non-24 hour sleep-wake disorder in adult patients and for the treatment of nighttime sleep disturbances in Smith-Magenis Syndrome in patients ≥16 years old. Tasimelteon oral suspension is indicated for the treatment of nighttime sleep disturbances in Smith-Magenis syndrome in patients 3 to 15 years of age. FDA Label Hetlioz is indicated for the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24) in totally blind adults. , Treatment of non-24-hour sleep-wake disorder in the totally blind Mechanism of Action Tasimelteon is a selective dual agonist of the melatonin receptors MT1 and MT2. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (10.19 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (10.19 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (10.19 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 5%DMSO + Corn oil: 2.0mg/ml (8.15mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0763 mL | 20.3815 mL | 40.7631 mL | |
| 5 mM | 0.8153 mL | 4.0763 mL | 8.1526 mL | |
| 10 mM | 0.4076 mL | 2.0382 mL | 4.0763 mL |