Physicochemical Properties
| Molecular Formula | C17H15CLF3NO2 |
| Molecular Weight | 357.7572 |
| Exact Mass | 357.074 |
| Elemental Analysis | C, 57.07; H, 4.23; Cl, 9.91; F, 15.93; N, 3.92; O, 8.94 |
| CAS # | 709676-56-2 |
| PubChem CID | 68453302 |
| Appearance | White to off-white solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 369.8±42.0 °C at 760 mmHg |
| Flash Point | 177.4±27.9 °C |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.553 |
| LogP | 5.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 24 |
| Complexity | 419 |
| Defined Atom Stereocenter Count | 0 |
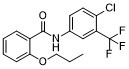
| SMILES | C(NC1=CC=C(Cl)C(C(F)(F)F)=C1)(=O)C1=CC=CC=C1OCCC |
| InChi Key | AQJBXYBDNZHZRE-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C17H15ClF3NO2/c1-2-9-24-15-6-4-3-5-12(15)16(23)22-11-7-8-14(18)13(10-11)17(19,20)21/h3-8,10H,2,9H2,1H3,(H,22,23) |
| Chemical Name | N-[4-chloro-3-(trifluoromethyl)phenyl]-2-propoxybenzamide |
| Synonyms | TTK-21; TTK21; TTK 21; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Enzyme Assay | Auto-acetylation reactions of full-length p300 were performed in lysine acetyltransferase assay buffer at 30°C for 10 min with or without TTK21, followed by the addition of 1 μl of 4.7 Ci/mmol [3H]acetyl-CoA (NEN–PerkinElmer). The reaction mixture was further incubated for another 10 min at 30°C. The 3H-labeled acetylated p300 was visualized by fluorography followed by autoradiography.[1] |
| Cell Assay |
One equivalent of SOCl2 diluted in DCM was added dropwise to a suspension of 100 mg of CSP in DCM, followed by the addition of few drops of DMF . The reaction mixture was stirred at room temperature for 8–9 h. TTK21 dissolved in DCM was added dropwise to this solution. The reaction mixture was stirred for 8–9 h at room temperature. The solvent was then evaporated and washed with cold water. The crude product was centrifuged and the supernatant (i.e., water) was removed; this procedure was repeated 7–8 times. Washing was then performed using DCM and the supernatant was subsequently tested for absence of TTK21. The CSP-TTK21 conjugated was then dried at 60°C for 2–3 d.[1] Highly purified HeLa core histones were incubated in HAT assay buffer at 30°C for 10 min with or without baculovirus-expressed recombinant p300 or CBP in the presence or absence of TTK21, followed by addition of 1 μl of 3.6 Ci/mmol [3H]acetyl-CoA (NEN–PerkinElmer) and incubated for 10 min in a final volume of 30 μl at 30°C.[1] |
| References |
[1],A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice PMID: 23804093 PMCID: PMC6618502 DOI: 10.1523/JNEUROSCI.5772-12.2013 [2],Reinstating plasticity and memory in a tauopathy mouse model with an acetyltransferase activator. PMID: 30275019 PMCID: PMC6220301 DOI: 10.15252/emmm.201708587 |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~279.52 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.99 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7952 mL | 13.9758 mL | 27.9517 mL | |
| 5 mM | 0.5590 mL | 2.7952 mL | 5.5903 mL | |
| 10 mM | 0.2795 mL | 1.3976 mL | 2.7952 mL |