TGR5 Receptor Agonist is a novel, potent and synthetic small molecule agonist of TGR5 (G-protein coupled receptor19, GPCR19), it showed improved potency in the U2-OS cell assay with pEC50 of 6.8 and in melanophore cells with pEC50 of 7.5. TGR5 Receptor Agonist is a novel, potent small molecule agonist of the human TGR5 G-protein coupled receptor. It is described as a 3-aryl-4-isoxazolecarboxamide analog found through a high-throughput screening campaign. TGR5 Receptor Agonist demonstrated improved GLP-1 secretion in vivo via an intracolonic dose coadministered with glucose challenge in a canine model. Treatments for metabolic diseases like type II diabetes and its aftereffects may benefit from targeting G-protein coupled receptors.
Physicochemical Properties
| Molecular Formula | C18H14CL2N2O2 | |
| Molecular Weight | 361.22 | |
| Exact Mass | 360.043 | |
| Elemental Analysis | C, 59.85; H, 3.91; Cl, 19.63; N, 7.76; O, 8.86 | |
| CAS # | 1197300-24-5 | |
| Related CAS # |
|
|
| PubChem CID | 44605616 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 541.8±50.0 °C at 760 mmHg | |
| Flash Point | 281.5±30.1 °C | |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C | |
| Index of Refraction | 1.627 | |
| LogP | 3.3 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 24 | |
| Complexity | 443 | |
| Defined Atom Stereocenter Count | 0 | |
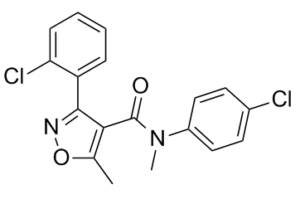
| SMILES | ClC1=CC=CC=C1C2=NOC(C)=C2C(N(C)C3=CC=C(Cl)C=C3)=O |
|
| InChi Key | IGRCWJPBLWGNPX-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C18H14Cl2N2O2/c1-11-16(17(21-24-11)14-5-3-4-6-15(14)20)18(23)22(2)13-9-7-12(19)8-10-13/h3-10H,1-2H3 | |
| Chemical Name | 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethyl-1,2-oxazole-4-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | GPCR19 ( EC50 = 7.5 ); GPCR19 ( pEC50 = 6.8 ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | TGR5 Receptor Agonist was tested in over 100 internal and external 7TM, ion channel, enzyme, transporter, and nuclear hormone receptor selectivity assays, including FXR, another bile acid receptor. It only demonstrated a statistically significant response in the pro-inflammatory cytokine TNFalpha secretion (pIC50 = 6.8) in human primary monocytes after stimulation with lipopolysaccharide (LPS). TGR5 Receptor Agonist additionally exhibits favorable physicochemical characteristics and exhibits no detectable activity against hERG dofetilide binding (pIC50<4.3) or any of the three common cytochrome P450 (CYP450) isoforms, 1A2, 2C9, and 2D6. | |
| Cell Assay | TGR5 Receptor Agonist demonstrated enhanced potency in melanophore cells (pEC50 of 7.5) and the U2-OS cell assay (pEC50 of 6.8). | |
| Animal Protocol |
Female C57BL/6J mice [12-18 weeks; TRPV1 knockout (trpv1-/-), TRPA1 knockout (trpa1-/-), or TGR5 knockout (Gpbar1-/-)] 100 µM, 100 µL Infused gently, to fill but not fully distend the bladder, and allowed to incubate for 5 min |
|
| References |
[1]. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. J Med Chem. 2009 Dec 24;52(24):7962-5. [2]. TGR5 agonists induce peripheral and central hypersensitivity to bladder distension. Sci Rep. 2022 Jun 15;12(1):9920. [3]. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021 Jul 6;33(7):1483-1492.e10. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 10 mg/mL (27.68 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 100.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 10 mg/mL (27.68 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 100.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7684 mL | 13.8420 mL | 27.6840 mL | |
| 5 mM | 0.5537 mL | 2.7684 mL | 5.5368 mL | |
| 10 mM | 0.2768 mL | 1.3842 mL | 2.7684 mL |