|
TCS PIM-1 1, also known as sc-204330, is a highly potent substituted pyridone and selective ATP-competitive inhibitor of Pim-1 kinase with IC50 of 50 nM, it displays good selectivity over Pim-2 and MEK1/MEK2(IC50s >20,000 nM). TCS PIM-1 1 bound convincingly within the ATP-binding site of Pim-1 suggesting an ATP-competitive inhibitory mechanism. Pim-1 kinase has been implicated in inflammatory bowel disease (IBD). Therefore, Pim-1 inhibitor (PIM-Inh) such as TCS PIM-1 1 has potential for the treatment of IBD. Structural requirements for in vitro activity are outlined as well as a complex crystal structure with TCS PIM-1 1. A hydrogen bond matrix involving the Pim-1 inhibitor, two water molecules, and the catalytic core, together with a potential weak hydrogen bond between an aromatic hydrogen on the R(1) phenyl ring and a main-chain carbonyl of Pim-1, accounts for the overall potency of this inhibitor.
|
Physicochemical Properties
| Molecular Formula |
C18H11BRN2O2
|
| Molecular Weight |
367.2
|
| Exact Mass |
366
|
| Elemental Analysis |
C, 58.88; H, 3.02; Br, 21.76; N, 7.63; O, 8.71
|
| CAS # |
491871-58-0
|
| Related CAS # |
|
| PubChem CID |
1235170
|
| Appearance |
Light yellow to yellow solid powder
|
| Density |
1.6±0.1 g/cm3
|
| Boiling Point |
592.6±50.0 °C at 760 mmHg
|
| Flash Point |
312.2±30.1 °C
|
| Vapour Pressure |
0.0±1.7 mmHg at 25°C
|
| Index of Refraction |
1.728
|
| LogP |
4.6
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
3
|
| Rotatable Bond Count |
2
|
| Heavy Atom Count |
23
|
| Complexity |
595
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
BrC1C([H])=C([H])C(=C(C=1[H])C1=C([H])C(=C(C#N)C(N1[H])=O)C1C([H])=C([H])C([H])=C([H])C=1[H])O[H]
|
| InChi Key |
SVSYJTYGPLVUOZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H11BrN2O2/c19-12-6-7-17(22)14(8-12)16-9-13(11-4-2-1-3-5-11)15(10-20)18(23)21-16/h1-9,22H,(H,21,23)
|
| Chemical Name |
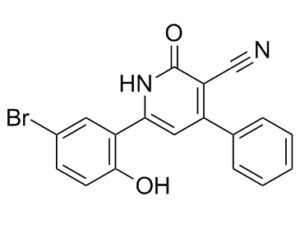
6-(5-bromo-2-hydroxyphenyl)-2-oxo-4-phenyl-1H-pyridine-3-carbonitrile
|
| Synonyms |
| TCS PIM 1 1; TCS-PIM-1 1; TCS PIM-1-1; SC-204330;SC 204330;SC204330 |
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder-20°C 3 years
4°C 2 years
In solvent -80°C 6 months
-20°C 1 month
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
|
Biological Activity
| ln Vitro |
Compound 1, or TCS PIM-1 1, is a substituted pyridone scaffold that binds to Pim-1's ATP-binding site with convincing force, indicating an ATP-competitive inhibitory mechanism. Additional preliminary data indicates that TCS PIM-1 1 did not exhibit any inhibitory activity in vitro against the related serine/threonine kinases MEK1/2 and Pim-2. As a result, tiny compounds like TCS PIM-1 1 could be helpful building blocks for the creation of additional, better, and more focused Pim-1 inhibitors. TCS PIM-1 1 was utilized for co-crystallization with Pim-1 protein and as a starting point for SAR chemical syntheses[1].
|
| ln Vivo |
| Mouse model of IBD was established by the treatment with trinitrobenzene sulphonic acid (TNBS). The results showed that disease activity index score was significantly decreased, colon length was significantly increased while Wallace score and pathological score were significantly decreased after PIM-Inh treatment compared to TNBS model group. In addition, GATA3 and ROR-γt mRNA and protein levels significantly increased but Foxp3 mRNA and protein levels significantly decreased in mice with TNBS treatment compared to mice without TNBS treatment. Administration of PIM-Inh caused significant decreases in GATA3, T-bet and ROR-γt mRNA and protein levels as well as significant increases in FOXP3 mRNA and protein levels. |
|
| Animal Protocol |
|
| References |
[1]. Identification and structure-activity relationships of substituted pyridones as inhibitors of Pim-1 kinase. Bioorg Med Chem Lett. 2007 Mar 15;17(6):1679-83.
|
| Additional Infomation |
3-cyano-4-phenyl-6-(3-bromo-6-hydroxyphenyl)-2-pyridone is a member of the class of pyridones that is 2-pyridone carrying cyano, phenyl and 3-bromo-6-hydroxyphenyl substituents at positions 3, 4 and 6 respectively It is a pyridone, a bromophenol and a nitrile.
|
|
Solubility Data
| Solubility (In Vitro) |
| DMSO:≥ 52 mg/mL | | Water:<1 mg/mL | | Ethanol:<1 mg/mL |
|
| Solubility (In Vivo) |
| OC1=C(C(N2)=CC(C3=CC=CC=C3)=C(C#N)C2=O)C=C(Br)C=C1 |
(Please use freshly prepared in vivo formulations for optimal results.)
|
| Preparing Stock Solutions |
|
1 mg |
5 mg |
10 mg |
| 1 mM |
2.7233 mL |
13.6166 mL |
27.2331 mL |
| 5 mM |
0.5447 mL |
2.7233 mL |
5.4466 mL |
| 10 mM |
0.2723 mL |
1.3617 mL |
2.7233 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles. |