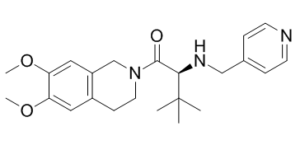
TCS-OX2-29 (TCS-OX229, TCS-OX-229), discovered from high throughput screening (HTS), is a potent, selective and non-peptide OX2 (orexin) receptor antagonist with potential usefulness in the treatment of insomnia. It inhibits OX2 with an IC50 of 40 nM and displays >250-fold selectivity for OX2 over OX1. Orexin receptor antagonists are expected to be a new approach for the treatment of insomnia that directly targets sleep/wake regulation. Several such compounds have entered into clinical development, including the dual orexin receptor antagonists, suvorexant and almorexant. TCS-OX2-29 shows selectivity for ion channels, and transporters (<30% inhibition at 10 μM), which includes G-protein coupled receptors associated with food intake including galanin and neuripeptide Y. TCS-OX2-29 Inhibits orexin A induced IP3 accumulation and ERK1/2 phosphorylation in CHO cells transfected with the OX2 receptor.
Physicochemical Properties
| Molecular Formula | C₂₃H₃₁N₃O₃ | |
| Molecular Weight | 397.51 | |
| Exact Mass | 397.24 | |
| Elemental Analysis | C, 69.49; H, 7.86; N, 10.57; O, 12.07 | |
| CAS # | 372523-75-6 | |
| Related CAS # | TCS-OX2-29 hydrochloride; 1610882-30-8 | |
| PubChem CID | 10408514 | |
| Appearance | White to off-white solid powder | |
| LogP | 4.318 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 29 | |
| Complexity | 530 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | O=C([C@]([H])(C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])N([H])C([H])([H])C1C([H])=C([H])N=C([H])C=1[H])N1C([H])([H])C2=C([H])C(=C(C([H])=C2C([H])([H])C1([H])[H])OC([H])([H])[H])OC([H])([H])[H] |
|
| InChi Key | COFVZFLCAOUMJT-OAQYLSRUSA-N | |
| InChi Code | InChI=1S/C23H31N3O3/c1-23(2,3)21(25-14-16-6-9-24-10-7-16)22(27)26-11-8-17-12-19(28-4)20(29-5)13-18(17)15-26/h6-7,9-10,12-13,21,25H,8,11,14-15H2,1-5H3/t21-/m1/s1 | |
| Chemical Name | (2S)-1-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-3,3-dimethyl-2-(pyridin-4-ylmethylamino)butan-1-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | OX2 Receptor; orexin-2 receptor (OX2R) (IC50 = 40 nM) | |
| ln Vitro |
|
|
| ln Vivo | TCS-OX2-29 (5-10 mg/kg; intraperitoneal injection; adult male NMRI mice) treatment significantly suppresses the acquisition and expression of conditioned place preference (CPP) in both naïve and dependent mice[2]. | |
| Enzyme Assay |
Full assay details are provided in the Supporting Information. 24-hour-old CHO cells that were seeded at a density of 25,000 cells/well and were stably expressing the human orexin-2 receptor were used for cell-based inositol phosphate and ERK1/2 phosphorylation functional assays in 96-well plates.
Radioligand binding [2] Cell membranes from HEK293 cells transiently expressing the human OX2 receptor (Supporting Information) were incubated with [3H]-EMPA in Krebs assay buffer (8.5 mM HEPES, 1.3 mM CaCl2, 1.2 mM MgSO4, 118 mM NaCl, 4.7 mM KCl, 4 mM NaHCO3, 1.2 mM KH2PO4, 11 mM glucose, pH 7.4) in a total assay volume of 0.25 mL with a final DMSO concentration of 1%. After 90 min incubation at room temperature, the reaction was terminated by rapid filtration through GF/B 96-well glass fibre plates with 5 × 0.25 mL washes with ddH2O using a Tomtec cell harvester. Bound radioactivity was determined through liquid scintillation using Lablogic SafeScint and detected on a microbeta liquid scintillation counter. Non-specific binding was determined as that remaining in the presence of a 10 μM saturating concentration of the antagonist EMPA. Saturation studies were carried out by incubating membranes (2 μg protein/well) with a range of concentrations of [3H]-EMPA (0.4 nM–15 nM). Radioligand concentrations were determined using SafeScint and a Beckman LS 6000 liquid scintillation counter. Competition binding was performed incubating membranes (2 μg protein/well) with 1.5 nM concentration of [3H]-EMPA and a range of concentrations of the test compound. Association kinetics for the radioligand were determined by adding the same cell membrane (2 μg protein/well) to wells containing Krebs buffer with 1% DMSO and 1.5 nM radioligand at various time points up to a total of 3 h. Dissociation kinetics were determined by pre-equilibrating membranes and [3H]-EMPA for 90 min; a saturating concentration of cold EMPA (100 μM) was then added at various time points to prevent re-association of the radioligand as it dissociates from the receptor. Kinetics of binding of unlabelled compounds was determined using the method of Motulsky and Mahan (1984) In brief, association curves for [3H]-EMPA in the absence or presence of three concentrations of competitive antagonist (typically 0.3, 1 and 3 x KI value). Association and dissociation rate constants for unlabelled compounds were determined by global analysis of the association data sets, as previously described (Dowling and Charlton, 2006); association and dissociation rate constants for [3H]-EMPA were fixed allowing the model to provide estimates of kon and koff for the test compounds. Ligand-receptor half-lives were calculated as 0.693/koff. Functional inositol phosphate and ERK1/2 phosphorylation assays [2] Cell-based inositol phosphate and ERK1/2 phosphorylation functional assays were performed in 96-well plates 24 h after seeding with CHO cells stably expressing the human orexin-2 receptor at a density of 25 000 cells/well; full assay details are in the Supporting Information. |
|
| Cell Assay |
In Krebs assay buffer (8.5 mM HEPES, 1.3 mM CaCl2, 1.2 mM MgSO4, 118 mM NaCl, 4.7 mM KCl, 4 mM NaHCO3, 1.2 mM KH2PO4, 11 mM glucose, pH 7.4), cell membranes from HEK293 cells transiently expressing the human OX2 receptor were incubated with [3H]-EMPA in a total assay volume of 0.25 mL with a final DMSO concentration of 1%. Using a Tomtec cell harvester, the reaction was quickly stopped after 90 minutes of room temperature incubation by filtering through GF/B 96-well glass fiber plates with 5 × 0.25 mL washes with ddH2O. Using Lablogic SafeScint for liquid scintillation, bound radioactivity was ascertained and detected on a microbeta liquid scintillation counter. The amount of non-specific binding was defined as that which persisted when the antagonist EMPA was present at a 10 μM saturating concentration. Membranes (2 μg protein/well) were incubated with a range of concentrations of [3H]-EMPA (0.4 nM–15 nM) in order to perform saturation studies. Using a Beckman LS 6000 liquid scintillation counter and SafeScint, radioligand concentrations were ascertained. In order to conduct competition binding, membranes (2 μg protein/well) were incubated with a range of concentrations of the test compound and 1.5 nM of [3H]-EMPA. Brief screening protocol: CHO-K1 cells stably expressing hOX1R or hOX2R were seeded into 96-well plates and incubated with a cytoplasmic calcium indicator, Fluo-3 AM. After the cells were washed four times, the intracellular Ca2+ mobilization evoked by 0.3 nM of orexins-A was monitored as a change in cell fluorescence intensity by FLIPR. Varying concentration of orexin antagonists were added to the plate 5 min prior to the addition of orexin-A. The antagonistic activities were calculated as IC50 values [1]. |
|
| Animal Protocol |
440 adult male NMRI mice (25-30 g) 5 mg/kg and 10 mg/kg Intraperitoneal injection (Pharmacokinetic study) |
|
| References |
[1]. N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: the first orexin-2 receptor selective non-peptidicantagonist. Bioorg Med Chem Lett, 2003 Dec 15, 13(24):4497-9. [2]. Binding kinetics differentiates functional antagonism of orexin-2 receptor ligands. Br J Pharmacol. 2014 Jan; 171(2): 351-363. |
|
| Additional Infomation |
The identification of potent and selective orexin-2 receptor (OX(2)R) antagonists is described based on the modification of N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline analogue 1, recently discovered during high throughput screening (HTS). Substitution of an acyl group in 1 with tert-Leucine (tert-Leu), and introduction of a 4-pyridylmethyl substituent onto the amino function of tert-Leu improved compound potency, selectivity, and water solubility. Thus, compound 29 is a promising tool to investigate the role of orexin-2 receptors.[1] Orexin receptor antagonism represents a novel approach for the treatment of insomnia that directly targets sleep/wake regulation. Several such compounds have entered into clinical development, including the dual orexin receptor antagonists, suvorexant and almorexant. In this study, we have used equilibrium and kinetic binding studies with the orexin-2 (OX₂) selective antagonist radioligand, [³H]-EMPA, to profile several orexin receptor antagonists. Furthermore, selected compounds were studied in cell-based assays of inositol phosphate accumulation and ERK-1/2 phosphorylation in CHO cells stably expressing the OX2 receptor that employ different agonist incubation times (30 and 5 min, respectively). EMPA, suvorexant, almorexant and TCS-OX-29 all bind to the OX₂ receptor with moderate to high affinity (pk(I) values ≥ 7.5), whereas the primarily OX1 selective antagonists SB-334867 and SB-408124 displayed low affinity (pK(I) values ca. 6). Competition kinetic analysis showed that the compounds displayed a range of dissociation rates from very fast (TCS-OX2-29, k(off) = 0.22 min⁻¹) to very slow (almorexant, k(off) = 0.005 min⁻¹). Notably, there was a clear correlation between association rate and affinity. In the cell-based assays, fast-offset antagonists EMPA and TCS-OX2-29 displayed surmountable antagonism of orexin-A agonist activity. However, both suvorexant and particularly almorexant cause concentration-dependent depression in the maximal orexin-A response, a profile that is more evident with a shorter agonist incubation time. Analysis according to a hemi-equilibrium model suggests that antagonist dissociation is slower in a cellular system than in membrane binding; under these conditions, almorexant effectively acts as a pseudo-irreversible antagonist.[2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.29 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.29 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.29 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5157 mL | 12.5783 mL | 25.1566 mL | |
| 5 mM | 0.5031 mL | 2.5157 mL | 5.0313 mL | |
| 10 mM | 0.2516 mL | 1.2578 mL | 2.5157 mL |