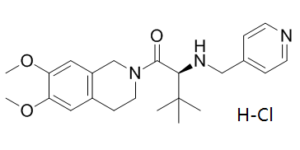
TCS-OX2-29 HCl, the hydrochloride salt of TCS-OX229, is a non-peptide and selective OX2 receptor antagonist (IC50 = 40 nM) with potential use in the treatment of insomnia.
Physicochemical Properties
| Molecular Formula | C23H32CLN3O3 | |
| Molecular Weight | 433.98 | |
| Exact Mass | 433.213 | |
| Elemental Analysis | C, 63.66; H, 7.43; Cl, 8.17; N, 9.68; O, 11.06 | |
| CAS # | 1610882-30-8 | |
| Related CAS # | TCS-OX2-29; 372523-75-6 | |
| PubChem CID | 53302033 | |
| Appearance | Solid powder | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 30 | |
| Complexity | 530 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | Cl.O=C(C(C(C)(C)C)NCC1C=CN=CC=1)N1CC2C=C(C(=CC=2CC1)OC)OC |
|
| InChi Key | NHKNHFJTMINMBP-ZMBIFBSDSA-N | |
| InChi Code | InChI=1S/C23H31N3O3.ClH/c1-23(2,3)21(25-14-16-6-9-24-10-7-16)22(27)26-11-8-17-12-19(28-4)20(29-5)13-18(17)15-26;/h6-7,9-10,12-13,21,25H,8,11,14-15H2,1-5H3;1H/t21-;/m1./s1 | |
| Chemical Name | (2S)-1-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-3,3-dimethyl-2-(pyridin-4-ylmethylamino)butan-1-one;hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | OX2 Receptor | |
| ln Vitro |
|
|
| ln Vivo |
TCS-OX2-29 (5-10 mg/kg; intraperitoneal injection; adult male NMRI mice) treatment significantly suppresses the acquisition and expression of conditioned place preference (CPP) in both naïve and dependent mice[3]. Conditioned place preference (CPP) has been associated with orexinergic (hypocrtinergic) system activation in naïve mice; however, the distinct role of different receptors of orexin in this paradigm has not been characterized yet. Moreover, the relationship between orexins and morphine in dependent mice may not be equal to naïve mice and seems noteworthy to investigate. We investigated the effects of systemic administration of orexin-1-receptor antagonist, SB 334867, and orexin-2 receptor antagonist, TCS-OX2-29 on the acquisition and expression of morphine conditioned place preference (CPP) in both naïve and morphine-dependent mice. We tested SB 334867 in three doses (10, 20 and 30 mg/kg), TCS-OX2-29 in two doses (5 and 10 mg/kg) and morphine with highest effective dose based on our dose-response experiment (5 mg/kg). Our results revealed that while SB 334867 suppressed CPP acquisition and expression in naïve mice, it failed to block CPP acquisition and expression in morphine dependent animals. In contrast, TCS-OX2-29 suppressed CPP acquisition and expression in both naïve and dependent mice significantly. The rewarding effect of morphine has stronger correlation with orexin-2 receptors in morphine-dependent mice while it depends on both kinds of receptors in naïve mice. This finding, if confirmed in other studies, persuades us to further investigate the role of orexin-2 receptor antagonists as potent drugs in addiction treatment.[2] |
|
| Enzyme Assay | Full assay details are provided in the Supporting Information. 24-hour-old CHO cells that were seeded at a density of 25,000 cells/well and were stably expressing the human orexin-2 receptor were used for cell-based inositol phosphate (Cisbio BioAssays, Codolet, France) and ERK1/2 phosphorylation (Surefire, PerkinElmer, Waltham, MA, USA) functional assays in 96-well plates | |
| Cell Assay | In Krebs assay buffer (8.5 mM HEPES, 1.3 mM CaCl2, 1.2 mM MgSO4, 118 mM NaCl, 4.7 mM KCl, 4 mM NaHCO3, 1.2 mM KH2PO4, 11 mM glucose, pH 7.4), cell membranes from HEK293 cells transiently expressing the human OX2 receptor (Supporting Information) were incubated with [3H]-EMPA in a total assay volume of 0.25 mL with a final DMSO concentration of 1%. Using a Tomtec cell harvester, the reaction was quickly stopped after 90 minutes of room temperature incubation by filtering through GF/B 96-well glass fiber plates with 5 × 0.25 mL washes with ddH2O. Utilizing Lablogic SafeScint for liquid scintillation, bound radioactivity was ascertained and detected using a microbeta liquid scintillation counter. The amount of non-specific binding was defined as that which persisted when the antagonist EMPA was present at a 10 μM saturating concentration. Membranes (2 μg protein/well) were incubated with a range of concentrations of [3H]-EMPA (0.4 nM–15 nM) in order to perform saturation studies. Using a Beckman LS 6000 liquid scintillation counter and SafeScint, radioligand concentrations were ascertained. In order to conduct competition binding, membranes (2 μg protein/well) were incubated with a range of concentrations of the test compound and 1.5 nM of [3H]-EMPA. | |
| Animal Protocol |
440 adult male NMRI mice (25-30 g) 5 mg/kg and 10 mg/kg Intraperitoneal injection (Pharmacokinetic study) |
|
| References |
[1]. N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: the first orexin-2 receptor selective non-peptidic antagonist. Bioorg Med Chem Lett. 2003 Dec 15;13(24):4497-9. [2]. The differential effects of OX1R and OX2R selective antagonists on morphine conditioned place preference in naïve versus morphine-dependent mice. Behav Brain Res. 2013 Jan 15;237:41-8. [3]. Binding kinetics differentiates functional antagonism of orexin-2 receptor ligands. Br J Pharmacol. 2014 Jan;171(2):351-63. |
|
| Additional Infomation | The identification of potent and selective orexin-2 receptor (OX(2)R) antagonists is described based on the modification of N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline analogue 1, recently discovered during high throughput screening (HTS). Substitution of an acyl group in 1 with tert-Leucine (tert-Leu), and introduction of a 4-pyridylmethyl substituent onto the amino function of tert-Leu improved compound potency, selectivity, and water solubility. Thus, compound 29 is a promising tool to investigate the role of orexin-2 receptors.[1] Orexin receptor antagonism represents a novel approach for the treatment of insomnia that directly targets sleep/wake regulation. Several such compounds have entered into clinical development, including the dual orexin receptor antagonists, suvorexant and almorexant. In this study, we have used equilibrium and kinetic binding studies with the orexin-2 (OX₂) selective antagonist radioligand, [³H]-EMPA, to profile several orexin receptor antagonists. Furthermore, selected compounds were studied in cell-based assays of inositol phosphate accumulation and ERK-1/2 phosphorylation in CHO cells stably expressing the OX2 receptor that employ different agonist incubation times (30 and 5 min, respectively). EMPA, suvorexant, almorexant and TCS-OX-29 all bind to the OX₂ receptor with moderate to high affinity (pk(I) values ≥ 7.5), whereas the primarily OX1 selective antagonists SB-334867 and SB-408124 displayed low affinity (pK(I) values ca. 6). Competition kinetic analysis showed that the compounds displayed a range of dissociation rates from very fast (TCS-OX2-29, k(off) = 0.22 min⁻¹) to very slow (almorexant, k(off) = 0.005 min⁻¹). Notably, there was a clear correlation between association rate and affinity. In the cell-based assays, fast-offset antagonists EMPA and TCS-OX2-29 displayed surmountable antagonism of orexin-A agonist activity. However, both suvorexant and particularly almorexant cause concentration-dependent depression in the maximal orexin-A response, a profile that is more evident with a shorter agonist incubation time. Analysis according to a hemi-equilibrium model suggests that antagonist dissociation is slower in a cellular system than in membrane binding; under these conditions, almorexant effectively acts as a pseudo-irreversible antagonist.[3] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3043 mL | 11.5213 mL | 23.0425 mL | |
| 5 mM | 0.4609 mL | 2.3043 mL | 4.6085 mL | |
| 10 mM | 0.2304 mL | 1.1521 mL | 2.3043 mL |