TBPB [1-(1'-2-methylbenzyl)-1,4'-bipiperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one] is a novel and highly selective allosteric agonist Muscarinic M1 receptor (mAChR) with EC50 of 289 nM. TBPB is completely agonist-inactive at all other mAChR subtypes, but it is very selective for the M(1) receptor. Studies on mutagenesis and molecular pharmacology have shown that TBPB activates M(1) via an allosteric site as opposed to an orthosteric acetylcholine binding site. This finding is probably important for the compound's exceptional selectivity. Whole-cell patch-clamp recordings showed that potentiating NMDA receptor currents in hippocampal pyramidal cells through activation of M(1) by TBPB does not modify excitatory or inhibitory synaptic transmission, which is believed to be mediated by M(2) and M(4). TBPB was effective at doses that did not cause catalepsy or the peripheral side effects of other mAChR agonists, in models predictive of antipsychotic-like activity in rats. Lastly, TBPB reduced the in vitro synthesis of Abeta and affected the processing of the amyloid precursor protein toward the non-amyloidogenic pathway. All of these findings point to a potential new treatment strategy for symptoms of schizophrenia and Alzheimer's disease: selective activation of M(1).
Physicochemical Properties
| Molecular Formula | C₂₅H₃₂N₄O | |
| Molecular Weight | 404.55 | |
| Exact Mass | 404.257 | |
| Elemental Analysis | C, 74.22; H, 7.97; N, 13.85; O, 3.95 | |
| CAS # | 634616-95-8 | |
| Related CAS # |
|
|
| PubChem CID | 10092649 | |
| Appearance | White to beige solid powder | |
| Density | 1.2±0.1 g/cm3 | |
| Index of Refraction | 1.623 | |
| LogP | 4.96 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 30 | |
| Complexity | 582 | |
| Defined Atom Stereocenter Count | 0 | |
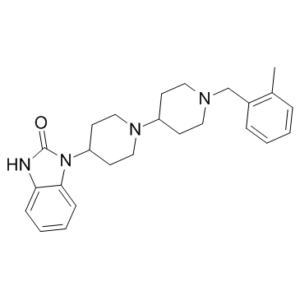
| SMILES | O=C1N(C2CCN(C3CCN(CC4=C(C)C=CC=C4)CC3)CC2)C5=CC=CC=C5N1 |
|
| InChi Key | CWPKTBMRVATCBL-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C25H32N4O/c1-19-6-2-3-7-20(19)18-27-14-10-21(11-15-27)28-16-12-22(13-17-28)29-24-9-5-4-8-23(24)26-25(29)30/h2-9,21-22H,10-18H2,1H3,(H,26,30) | |
| Chemical Name | 3-[1-[1-[(2-methylphenyl)methyl]piperidin-4-yl]piperidin-4-yl]-1H-benzimidazol-2-one | |
| Synonyms | TBPB | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | mAChR1 | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | TBPB is a newly developed, highly selective allosteric agonist that binds to the Muscarinic M1 receptor (mAChR) at an EC50 of 289 nM. Studies on mutagenesis and molecular pharmacology have shown that TBPB activates M(1) through an allosteric site rather than the orthosteric acetylcholine binding site, which is likely crucial for its unparalleled selectivity. TBPB is highly selective for the M(1) receptor with no agonist activity at any of the other mAChR subtypes. | ||
| Cell Assay | Whole-cell patch-clamp recordings showed that potentiating NMDA receptor currents in hippocampal pyramidal cells through activation of M(1) by TBPB does not modify excitatory or inhibitory synaptic transmission, which is believed to be mediated by M(2) and M(4). At doses that did not result in catalepsy or the peripheral side effects of other mAChR agonists, TBPB was effective in models indicative of antipsychotic-like activity in rats. In conclusion, TBPB impacted the processing of the amyloid precursor protein towards the non-amyloidogenic route and reduced the in vitro production of Abeta. Collectively, these findings imply that selective M(1) activation might offer a novel strategy for treating symptoms linked to schizophrenia and Alzheimer's disease. | ||
| Animal Protocol |
|
||
| References |
[1]. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008 Oct 8;28(41):10422-33. [2]. Synthesis and SAR of analogs of the M1 allosteric agonist TBPB. Part II: Amides, sulfonamides and ureas--the effect of capping the distal basic piperidine nitrogen. Bioorg Med Chem Lett. 2008 Oct 15;18(20):5443-7. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (6.18 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (6.18 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4719 mL | 12.3594 mL | 24.7188 mL | |
| 5 mM | 0.4944 mL | 2.4719 mL | 4.9438 mL | |
| 10 mM | 0.2472 mL | 1.2359 mL | 2.4719 mL |