Physicochemical Properties
| Molecular Formula | C20H20O7 |
| Molecular Weight | 372.3686 |
| Exact Mass | 372.12 |
| CAS # | 2306-27-6 |
| PubChem CID | 145659 |
| Appearance | White to light yellow solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 547.8±50.0 °C at 760 mmHg |
| Melting Point | 174-176ºC |
| Flash Point | 240.6±30.2 °C |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.566 |
| LogP | 3.08 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 27 |
| Complexity | 548 |
| Defined Atom Stereocenter Count | 0 |
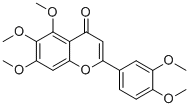
| SMILES | O1C(=C([H])C(C2=C(C(=C(C([H])=C12)OC([H])([H])[H])OC([H])([H])[H])OC([H])([H])[H])=O)C1C([H])=C([H])C(=C(C=1[H])OC([H])([H])[H])OC([H])([H])[H] |
| InChi Key | LKMNXYDUQXAUCZ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C20H20O7/c1-22-13-7-6-11(8-15(13)23-2)14-9-12(21)18-16(27-14)10-17(24-3)19(25-4)20(18)26-5/h6-10H,1-5H3 |
| Chemical Name | 2-(3,4-dimethoxyphenyl)-5,6,7-trimethoxychromen-4-one |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | In the absence of IBMX, sinesetin (40 μM, 2 d) modifies adipogenic factors, promoting adipogenesis in 3T3-L1 preadipocytes [1]. On Jurkat and CCRF-CEM cells, sinesetin (12-200 μM, 24-48 h) shows notable cytotoxic effects in a dose- and time-dependent manner [4]. Jurkat cells undergo sub-G1 phase induction and cell transplantation when exposed to 100 μM sinensetin for 48 hours [4]. Assay [1] |
| ln Vivo | Sinensetin (50 mg/kg, single dose, intraperitoneal injection) exerts anti-inflammatory properties in a carrageenan-induced mouse paw activation model [5]. |
| Cell Assay |
Western Blot Analysis[1] Cell Types: 3T3-L1 Tested Concentrations: 2, 10, 40 μM Incubation Duration: 24 days Experimental Results: Cellular lipid accumulation and triglyceride content increased in a dose-dependent manner. The expression of PPARγ1, PPARγ2, C/EBPα, and aP2 was increased. Cell proliferation assay [4] Cell Types: CCRF-CEM cells, Jurkat Tested Concentrations: 6.25–100 μM Incubation Duration: 24 or 48 hrs (hours) Experimental Results: Different concentrations of artemisia inhibited cell viability for 24 hrs (hours) and 48 hrs (hours). Apoptosis analysis [4] Cell Types: Jurkat Cell Tested Concentrations: 50 μM, 100 μM Incubation Duration: 24 hrs (hours) and 48 hrs (hours) Experimental Results: Induction of sub-G1 population and apoptosis. |
| Animal Protocol |
Animal/Disease Models: Carrageenan-induced paw edema in male C57BL/6 mice [5] 50 mg/kg, single Doses: intraperitoneal (ip) injection Experimental Results: Carrageenan-treated paw volume at 6 hrs (hrs (hours)) Increase slows down. |
| References |
[1]. Kang SI et al. Sinensetin enhances adipogenesis and lipolysis by increasing cyclic adenosine monophosphate levels in 3T3-L1 adipocytes. Biol Pharm Bull. 2015;38(4):552-8. [2]. Shin HS et al. Sinensetin attenuates LPS-induced inflammation by regulating the protein level of IκB-α. Biosci Biotechnol Biochem. 2012;76(4):847-9. [3]. Lam IK et al. In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: identifying sinensetin as a novel antiangiogenesis agent. Mol Nutr Food Res. 2012 Jun;56(6):945-56. [4]. Kok-Tong Tan, et al. Sinensetin induces apoptosis and autophagy in the treatment of human T-cell lymphoma. Anticancer Drugs. 2019, 30, 5. [5]. Mirka Laavola, et al. Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med. 2012, 78, 8. |
| Additional Infomation |
Sinensetin is a pentamethoxyflavone that is flavone substituted by methoxy groups at positions 5, 6, 7, 3' and 4' respectively. It has a role as a plant metabolite. It is functionally related to a flavone. Sinensetin has been reported in Citrus leiocarpa, Citrus myrtifolia, and other organisms with data available. See also: Tangerine peel (part of); Citrus aurantium fruit rind (part of). |
Solubility Data
| Solubility (In Vitro) | DMSO : ~50 mg/mL (~134.28 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.25 mg/mL (3.36 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.25 mg/mL (3.36 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6855 mL | 13.4275 mL | 26.8550 mL | |
| 5 mM | 0.5371 mL | 2.6855 mL | 5.3710 mL | |
| 10 mM | 0.2686 mL | 1.3428 mL | 2.6855 mL |