Physicochemical Properties
| Molecular Formula | C44H56N8O7 |
| Molecular Weight | 808.96 |
| Exact Mass | 808.427 |
| Elemental Analysis | C, 64.92; H, 6.87; N, 13.17; O, 15.04 |
| CAS # | 81377-02-8 |
| Related CAS # | 99248-33-6 (Seglitide acetate) |
| PubChem CID | 5311430 |
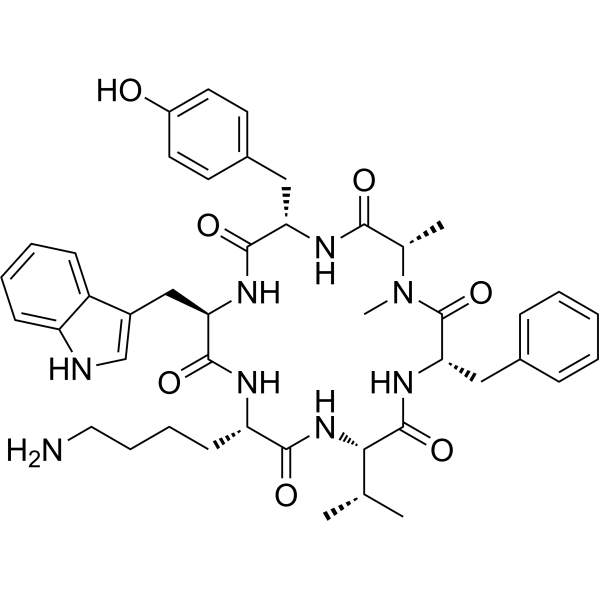
| Sequence | cyclo[N-methyl-L-alanyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-phenylalanyl]; Cyclo({Ala(Me)}-Tyr-{d-Trp}-Lys-Val-Phe) |
| SequenceShortening | cyclo[N(Me)Ala-Tyr-D-Trp-Lys-Val-Phe]; Cyclo({Ala(Me)}-Y-{d-Trp}-KVF) |
| Appearance | Typically exists as solid at room temperature |
| Index of Refraction | 1.569 |
| LogP | 4.253 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 59 |
| Complexity | 1430 |
| Defined Atom Stereocenter Count | 6 |
| SMILES | C([C@@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC2C=CC=CC=2)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CC2C=CC(O)=CC=2)C(=O)N1)C1=CNC2C=CC=CC1=2 |
| InChi Key | NPJIOCBFOAHEDO-AVWFULIKSA-N |
| InChi Code | InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55)/t27-,34-,35-,36+,37-,38-/m0/s1 |
| Chemical Name | (3S,6S,9S,12R,15S,18S)-9-(4-aminobutyl)-3-benzyl-15-[(4-hydroxyphenyl)methyl]-12-(1H-indol-3-ylmethyl)-1,18-dimethyl-6-propan-2-yl-1,4,7,10,13,16-hexazacyclooctadecane-2,5,8,11,14,17-hexone |
| Synonyms | MK-678; Seglitide acetate [USAN]; UNII-URM20C99DG; URM20C99DG; MK-678; Seglitide (acetate); cyclo(MATTLVP); L-363586 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | SSTR2/somatostatin receptor 2 |
| ln Vitro | Researchers report that in guinea-pig isolated right atria, seglitide, a potent cyclic hexapeptide somatostatin agonist, behaves as a competitive somatostatin receptor antagonist with pA2 values against SS14, SS25 and SS28, of 6.50 +/- 0.40, 6.24 +/- 0.08 and 6.09 +/- 0.06, respectively. Seglitide had little or no effect on the negative inotropic action of carbachol or N6-cyclohexyladenosine. Our findings indicate that the receptor-response coupling characteristics of guinea-pig atria are such that in this preparation seglitide has low intrinsic activity and behaves specifically as a somatostatin receptor antagonist.[1] |
| ln Vivo | A hypotensive effect of an orally-administered cyclopeptide somatostatin analog, MK-678, has been demonstrated in a hypertensive diabetic rat model. Sustained blood pressure reduction failed to occur when the drug was administered to the spontaneously hypertensive rat. The mechanism of hypotension appears independent of effects on a variety of hormones including insulin, glucagon, growth hormone, and components of the renin-angiotensin system including renin activity, plasma angiotensin converting enzyme, and aldosterone.[3] |
| Animal Protocol |
Histochemistry of sstr2 expression [2] Six adult male and six female sstr2+/lacZ mice were perfused with 3% PFA and coronal sections through the MS, rPOA, and AH were processed for X-gal histochemistry by washing in TBS followed by X-gal solution [2 mM MgCl2, 4 mM K3Fe(CN)6, 4 mM K4Fe(CN)6, and 4 mg/ml 5-bromo-4-chloro-3-indoyl-β-D-galactosidase in TBS] overnight at room temperature to reveal lacZ-expressing cells within the brain sections. Sections were then processed for GnRH immunocytochemistry using an LR1 antibody and diaminobenzidine chromogen as detailed previously. GnRH neurons located in MS, rPOA, and AH were examined. Two sections from each brain region were selected, and the numbers of single (GnRH) and double-labeled (GnRH plus X-gal staining) neurons were determined. The X-gal expression in GnRH-immunoreactive neurons was calculated as the percentage of total number of GnRH-immunoreactive neurons in each region. Brain slice preparation and electrophysiology [2] Brain slices were acutely prepared as described (18). Mice were decapitated and brains rapidly removed and placed in the ice-cold bicarbonate-buffered artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 11 D-glucose, 1.4 NaH2PO4, and 25 NaHCO3 (pH 7.4 when bubbled with 95% O2 and 5% CO2). Brains were blocked and glued with cyanoacrylate to the chilled stage of a vibratome, and 150- to 200-μm-thick coronal slices containing the rPOA were cut. The slices were placed in oxygenated ACSF for at least 1 h at room temperature. The slices were transferred to the recording chamber, held submerged, and continuously superfused with ACSF at a rate of 4–5 ml/min. The slices were viewed with an upright microscope and fluorescent GnRH neurons identified at ×10 and ×40 objective magnification by brief fluorescence illumination and then viewed and patched under Nomarski differential interference contrast optics. Patch pipettes were pulled from thin-wall borosilicate glass-capillary tubing on a Flaming/Brown Micropipette puller. The pipette solution was passed through a disposable 0.22-μm filter and contained (in mM) 130 KCl, 5 NaCl, 0.4 CaCl2, 1 MgCl2, 10 HEPES, and 1.1 EGTA (pH 7.3 with KOH). Gramicidin was first dissolved in dimethylsulfoxide to a concentration of 2.5–5 mg/ml and then diluted in the pipette solution just before use to a final concentration of 2.5–5 μg/ml and sonicated for 10 min. The gramicidin-perforated patch recordings were performed using an Axopatch 200B amplifier. The tip resistance of the electrode was 4–6 Mohm. In initial experiments, access resistance was monitored and experiments begun when resistance stabilized at 50–90 Mohm. This typically took 15–20 min after gigaseal formation and always corresponded to the resting membrane potential (RMP) of the cell reaching a stable level below −45 mV. In all subsequent cells, experiments were begun when the RMP reached a stable level below −45 mV. Spontaneous rupture of the seal was evident by a sudden overshooting of action potentials above 0 mV. Membrane potential changes were sampled online using a Digidata 1322A interface connected to an IBM PC. Any GnRH neuron that displayed a shift in resting membrane potential of more than 2 mV was considered to have responded. Acquisition and subsequent analysis of the acquired data were performed using the Clampex9 software. Traces were plotted using the Origin7 software. All recordings were made at room temperature. |
| References |
[1]. Antagonist effects of seglitide (MK 678) at somatostatin receptors in guinea-pig isolated right atria. Br J Pharmacol. 1993;109(4):898-899. [2]. Somatostatin inhibition of gonadotropin-releasing hormone neurons in female and male mice[J]. Endocrinology, 2010, 151(7): 3258-3266. [3]. Blood pressure reduction in hypertensive-diabetic rats by the somatostatin analog MK-678. Life Sci. 1989;45(3):267-74. |
| Additional Infomation |
Previous studies indicate that somatostatin regulates gonadotropin secretion. We investigated here whether somatostatin has direct effects on GnRH neurons in the adult male and female mice. Dual-labeling immunofluorescence experiments revealed the presence of somatostatin-immunoreactive fibers adjacent to GnRH neurons, and three-dimensional confocal reconstructions demonstrated apparent somatostatin fiber appositions with 50-60% of GnRH neurons located throughout the brain in both male and female mice. Perforated patch-clamp recordings from GnRH-green fluorescent protein neurons revealed that approximately 70% of GnRH neurons responded in a dose-dependent manner to 10-300 nm somatostatin with an acute membrane hyperpolarization and cessation of firing. This effect persisted in the presence of tetrodotoxin and amino acid receptor antagonists, indicating a direct postsynaptic site of action on the GnRH neuron. The identity of the somatostatin receptors underlying this action was assessed using GnRH neuron single-cell RT-PCR. Of the somatostatin receptor subtypes, the sstr2 transcript was the most prevalent and detected in both males and females. The expression of sstr2 by GnRH neurons was confirmed in the sstr2 knockout/LacZ knock-in mouse line. Electrophysiological studies demonstrated that the sstr2-selective agonist seglitide exerted acute hyperpolarizing actions on GnRH neurons identical to those of somatostatin. Together, these studies reveal somatostatin, acting through sstr2, to be one of the most potent inhibitors of electrical excitability of male and female GnRH neurons identified thus far.[2]
Our RT-PCR and histochemical studies suggest that sstr2 is the most prevalent somatostatin receptor subtype in GnRH neurons in both sexes. Our results are also in good agreement with Todman et al., who demonstrated that 25% of GnRH neurons express sstr2 in female mice using single-cell microarray on GnRH neurons. Here we further investigated whether sstr2 is involved in the somatostatin-induced inhibitory effects. Seglitide, an sstr2-specific agonist, mimicked the somatostatin-induced membrane hyperpolarization and was maintained in the presence of TTX and the amino acid receptor antagonist cocktail, suggesting a direct action on GnRH neurons. These results demonstrate that sstr2 is very likely expressed postsynaptically in the GnRH neuron and can mediate membrane hyperpolarization in response to somatostatin on GnRH neurons. Interestingly, gene profiling experiments with heterozygous sstr2+/lacZ mice and RT-PCR showed that only 10–20% of GnRH neurons transcribe sstr2 or express sstr2 mRNA, whereas single-cell electrophysiological investigations indicate that 50% of GnRH neurons are suppressed by seglitide, a specific antagonist for sstr2 (IC50/Kd, 0.2–1.5 nM). Although the IC50/Kd values of seglitide for the cloned human sstr5 show a relatively wide range (0.06–23 nM, GnRH neurons do not express sstr5, ruling out the activation of these receptors on GnRH neurons after seglitide administration. The discrepancy between the results of the gene profiling (10–20% expression) and electrophysiological (50–70% expression) investigations are most likely explained by differences in detection sensitivity of these methods, with electrophysiological approaches being the most sensitive index of functional receptor expression.[2] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2362 mL | 6.1808 mL | 12.3616 mL | |
| 5 mM | 0.2472 mL | 1.2362 mL | 2.4723 mL | |
| 10 mM | 0.1236 mL | 0.6181 mL | 1.2362 mL |