STA-21 (NSC628869; Ochromycinone; STA21; Rac-STA-21) is a potent STAT3 inhibitor and natural antibiotic with anticancer and antimicrobial activity. It inhibits STAT3 with IC50 of 12.2 μM in DU145 cells. In cells, STA-21 inhibits Stat3 DNA binding activity, Stat3 dimerization, and Stat3-dependent luciferase activity. STA-21 remarkably inhibits the growth and the survival of the breast carcinoma cells MDA-MB-231, MDA-MB-435s, and MDA-MB-468 that express persistently activated Stat3. In RH30 and RD2 cells, STA-21 also inhibits cell viability and growth and induced apoptosis through caspases 3, 8 and 9 pathways
Physicochemical Properties
| Molecular Formula | C19H14O4 | |
| Molecular Weight | 306.31 | |
| Exact Mass | 306.089 | |
| CAS # | 111540-00-2 | |
| Related CAS # | (+)-Ochromycinone;28882-53-3 | |
| PubChem CID | 363709 | |
| Appearance | Light yellow to yellow solid powder | |
| LogP | 2.932 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 0 | |
| Heavy Atom Count | 23 | |
| Complexity | 554 | |
| Defined Atom Stereocenter Count | 0 | |
| InChi Key | ZAWXOCUFQSQDJS-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C19H14O4/c1-9-7-10-5-6-12-17(15(10)14(21)8-9)19(23)11-3-2-4-13(20)16(11)18(12)22/h2-6,9,20H,7-8H2,1H3 | |
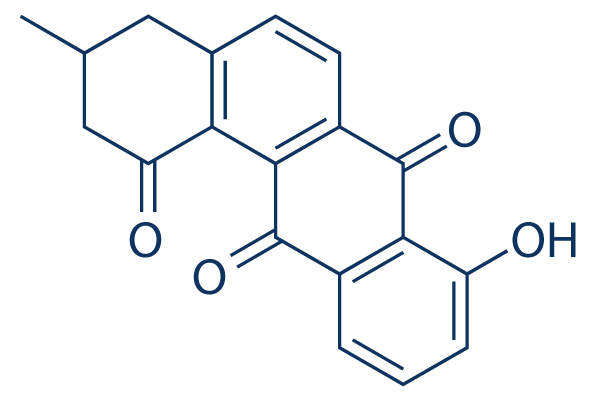
| Chemical Name | 8-hydroxy-3-methyl-3,4-dihydrotetraphene-1,7,12(2H)-trione | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | STAT3 dimerization, STAT3-dependent luciferase activity, and STAT3 DNA binding activity are all inhibited by STA-21. Moreover, STA-21 decreased the survival of breast cancer cells that expressed constitutive STAT3 signaling, while it had no effect on those that did not [2]. | ||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References |
[1]. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4700-5. [2]. New Angucyclinones from the Marine Mollusk Associated Actinomycete Saccharothrix espanaensis An 113. Natural product communications. 2008, 3(10):1611-1616. |
||
| Additional Infomation | See also: Ochromycinone (annotation moved to). |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.67 mg/mL (5.45 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2647 mL | 16.3233 mL | 32.6467 mL | |
| 5 mM | 0.6529 mL | 3.2647 mL | 6.5293 mL | |
| 10 mM | 0.3265 mL | 1.6323 mL | 3.2647 mL |