SR-11302 (SR11302) is an AP-1 (activator protein-1) transcription factor inhibitor with antitumor activity. SR11302 does not activate transcription from the retinoic acid response element (RARE) and displays no activity at retinoic acid receptors (EC50 > 1 μM for RARα, RARβ, RARγ and RXRα).
Physicochemical Properties
| Molecular Formula | C26H32O2 |
| Molecular Weight | 376.53108 |
| Exact Mass | 376.24 |
| CAS # | 160162-42-5 |
| PubChem CID | 9976842 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.048g/cm3 |
| Boiling Point | 541.567ºC at 760 mmHg |
| Flash Point | 414.977ºC |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.587 |
| LogP | 7.048 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 28 |
| Complexity | 705 |
| Defined Atom Stereocenter Count | 0 |
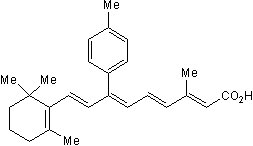
| SMILES | CC1=C(C(CCC1)(C)C)/C=C/C(=C/C=C/C(=C/C(=O)O)/C)/C2=CC=C(C=C2)C |
| InChi Key | RQANARBNMTXCDM-DKOHIBGUSA-N |
| InChi Code | InChI=1S/C26H32O2/c1-19-11-13-23(14-12-19)22(10-6-8-20(2)18-25(27)28)15-16-24-21(3)9-7-17-26(24,4)5/h6,8,10-16,18H,7,9,17H2,1-5H3,(H,27,28)/b8-6+,16-15+,20-18+,22-10- |
| Chemical Name | (2E,4E,6Z,8E)-3-methyl-7-(4-methylphenyl)-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Strong anti-AP-1 activity is exhibited by SR 11302 (SR11302), which selectively binds to RARα and RARγ but not to RARβ or RXRα [1]. In cells treated with hypoxia, SR 11302 (SR-11302; 1 μM) decreases aldosterone levels by 61.9% and inhibits the activity of the AP-1 transcription factor [2]. Helicobacter pylori (H. pylori)-induced gastric adenocarcinoma (AGS) cell proliferation is inhibited by SR 11302 (SR-11302; 2 μM; 48 hours) [3]. In AGS cells, β-catenin and c-myc expression generated by Helicobacter pylori is inhibited by SR 11302 (2 μM; 24 hours) [3]. |
| ln Vivo | Treatment with SR 11302 (SR11302; low dosage 0.5 mg/kg and high dose 1 mg/kg body weight; daily oral gavage) lowers total vascular lesion number and lesion size in Vldlr-/- mice in a dose-dependent manner [4] . |
| Animal Protocol |
Animal/Disease Models: Vldlr-/- mice [4] Doses: low dose 0.5 mg/kg and high dose 1 mg/kg Body weight Route of Administration: P5 to P15 Daily po (oral gavage) Experimental Results: P5 to P15 High dose reduces total vascular lesions The number was diminished by 48%, the lesion size was diminished by 40%, and no signs of toxicity were detected in the mice, including no changes in body weight. |
| References |
[1]. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A. 1997 May 27;94(11):5826-30. [2]. Upregulation of steroidogenic acute regulatory protein by hypoxia stimulates aldosterone synthesis in pulmonary artery endothelial cells to promote pulmonary vascular fibrosis. Circulation. 2014 Jul 8;130(2):168-79. [3]. Activation of NF-κB and AP-1 Mediates Hyperproliferation by Inducing β-Catenin and c-Myc in Helicobacter pylori-Infected Gastric Epithelial Cells. Yonsei Med J. 2016 May;57(3):647-51. [4]. Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c-Fos. J Exp Med. 2017 Jun 5;214(6):1753-1767. |
| Additional Infomation | SR11302 is a retinoid that is all-trans-retinoic acid in which the methyl group at position 9 is replaced by a 4-methylphenyl group. It is an inhibitor of activator protein-1 which exhibits antitumour effects in vivo. It has a role as an AP-1 antagonist and an antineoplastic agent. It is a retinoid, a member of toluenes and an alpha,beta-unsaturated monocarboxylic acid. It is functionally related to an all-trans-retinoic acid. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~25 mg/mL (~66.40 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (6.64 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: 2 mg/mL (5.31 mM) in 1% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6558 mL | 13.2792 mL | 26.5583 mL | |
| 5 mM | 0.5312 mL | 2.6558 mL | 5.3117 mL | |
| 10 mM | 0.2656 mL | 1.3279 mL | 2.6558 mL |