Physicochemical Properties
| Molecular Formula | C22H28CLNO7 |
| Molecular Weight | 453.91 |
| Exact Mass | 453.155 |
| CAS # | 155059-42-0 |
| PubChem CID | 11691005 |
| Appearance | Typically exists as solid at room temperature |
| LogP | 3.315 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 31 |
| Complexity | 516 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | CCC(C(=O)OC1CC2CCC(C1)N2C)OC3=CC=C(C=C3)Cl.C(=C\C(=O)O)\C(=O)O |
| InChi Key | BHXGTFUQDGMXHA-BTJKTKAUSA-N |
| InChi Code | InChI=1S/C18H24ClNO3.C4H4O4/c1-3-17(22-15-8-4-12(19)5-9-15)18(21)23-16-10-13-6-7-14(11-16)20(13)2;5-3(6)1-2-4(7)8/h4-5,8-9,13-14,16-17H,3,6-7,10-11H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;2-1- |
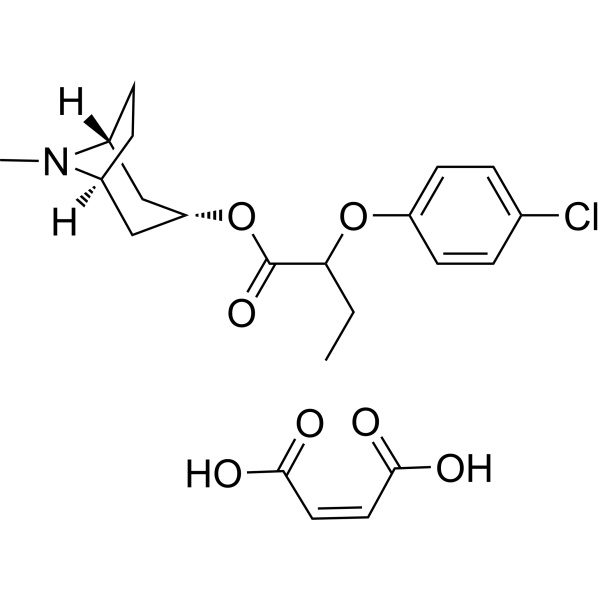
| Chemical Name | (Z)-but-2-enedioic acid;(8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 2-(4-chlorophenoxy)butanoate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Central muscarinic receptors; Sigma-1 receptor |
| ln Vitro | Several analogs of the alpha-tropanyl esters of 2-(4-chlorophenoxy)butyric acid (SM21) and 2-phenylthiobutyric acid (SM32), endowed with potent antinociceptive and cognition enhancing activity, were synthesized, aimed at obtaining more potent and safe drug candidates. Variation of the acyl moiety (4-11), as well as the conformational restriction of atropine to give the alpha-tropanyl ester of 2,3-dihydrobenzofurane-3-carboxylic acid (18), practically abolished activity. In the case of 18, the antimuscarinic activity was also severely affected by the conformation restrain. On the contrary, conformational restriction of phenoxybutyric and phenylthiobutyric acid derivatives to give the alpha-tropanyl ester of 2,3-dihydro-benzofurane-2-carboxylic acid and 2,3-dihydro-benzothiophene-2-carboxylic acid (12-17), afforded potent analgesic drugs that unfortunately were too toxic to be reliable drug candidates. A series of related esters of benzofurane-3-carboxylic acid (20-27) and benzothiophene-3-carboxylic acid (28) were also studied and found to be potent but toxic analgesics [1]. |
| ln Vivo |
As a matter of fact, the analgesic activity of 15 at the maximal effect dose (MED=50 mg/kg) is almost completely reversed (66%) by SDZ-205557, a 5-HT4 antagonist. The reversal of the analgesic activity of SM21 (2) was, under the same conditions, quite lower (44%). We may conclude that conformational restriction of atropine, SM21 and SM32, apparently and contrary to our expectations, favors interaction with the 5-HT4, respect to presynaptic muscarinic receptors. [1]
Further modifications of the leads ((R)-(+)-hyoscyamine and (p-chlorophenyl)propionic acid alpha-tropanyl ester), which show analgesic and nootropic activities as a consequence of increased central presynaptic ACh release, are reported. 2-Phenoxy- and 2-(phenylthio)alkanoic acid esters showed the best results. Several members of these classes possess analgesic properties which are comparable to that of morphine and at the same time are able to reverse dicyclomine-induced amnesia. Confirmation was found that the mechanism of action is due to an increase in ACh release at central muscarinic synapses and that both auto- and heteroreceptors controlling ACh release are very likely involved. According to the results obtained with (R)-(+)-hyoscyamine, analgesic activity is stereochemistry dependent, since the R-(+)-enantiomers are always more efficacious than the corresponding S-(-)-ones. On the basis of their potency and acute toxicity, compounds (+/-)-28 (SM21) and (+/-)-42 (SM32) were selected for further study [2]. |
| Animal Protocol |
Analgesic activity [1] The plate temperature was fixed at 52.5±0.1°C. An arbitrary cutoff time of 45 s was adopted. The number of mice treated in each test varied from 8 to 20. The level of analgesia reached was evaluated comparing the analgesic effect of the maximal effect dose of each compound to that of morphine, taken as the reference compound, and injected at 8 mg/kg s.c., a dose that does not alter animal behavior. Calculations were performed using the following formula: analgesic efficacy of X expressed as percentage of that of morphine · HCl (8 mg/kg s.c.)=(maximum reaction time of X−pretest reaction of X)/(maximum reaction time of morphine−pretest reaction of morphine)×100. Standard errors (SEs) on the values expressed as percentage were not evaluated. Original data however, have been statistically processed by employing Dunnett’s two-tailed test in order to verify the significance of the differences between the means shown by treated mice at the maximum reaction time and the pretest reaction time. Differences were considered statistically significant when P≤0.05. Percent values were calculated only for those differences that were statistically significant; in all other cases, the drugs were considered inactive. Since the reaction time was measured with an accuracy of ±15%, the errors on the percent values calculated through the formula reported above are in the same range. Antimuscarinic activity [1] Male guinea pigs (200–300 g), female guinea pigs (150–200 g) and male New Zealand white rabbits (2.5–3 kg) were killed by cervical dislocation and the organs were set up under the appropriate tension (see below) in 13 ml organ baths containing physiological salt solution (PSS) kept at an appropriate temperature (see below) and treated with 5% CO2–95% O2. Dose–response curves were constructed by addition of the agonist (cumulative curves in the case of rabbit vas deferens and guinea pig atria). The concentration of agonist in the organ bath was increased approximately 3-fold each step, each addition being made only after the response to the previous addition had attained a maximal level and remained steady. Tissues were incubated with the antagonist for 1 h and a new dose–response curve to the agonist was obtained. Contractions were recorded by means of a force transducer connected to a single channel recorder (U. Basile). Rabbit stimulated vas deferens [1] Surrounding tissues were carefully removed from vasa deferentia which were then divided into four segments, two prostatic portions of 1 cm and two epididymal portions approximately 1.5 cm long. The four segments were mounted under 0.75 g tension in PSS with the following composition (mM): NaCl (118.4), KCl (4.7), CaCl2 (1.8), MgCl2 (0.6), KH2PO4 (1.18), NaHCO3 (25), glucose (11.1); 10−6 M yohimbine was included to block α2-adrenoceptors. The solution was maintained at 32°C and tissues were stimulated through platinum electrodes by square-wave pulses (2 ms, 0.1 Hz, 10–30 V). Contractions were measured isometrically after tissues had been equilibrated for 1 h, then a cumulative dose–response curve for the inhibitory effect of McN-A-343 was plotted. Guinea pig stimulated left atria [1] The heart was rapidly removed and the left atria were excised and mounted under 1 g of tension in PSS with the following composition (mM): NaCl (137), KCl (2.7), CaCl2 (1.8), MgCl2 (1.05), NaH2PO4 (0.42), NaHCO3 (11.9), glucose (5.6). The solution was maintained at 30°C and stimulated through platinum electrodes by square-wave pulses (1 ms, 1 Hz, 4–10 V). Inotropic activity was recorded isometrically. Tissues were equilibrated for 1 h and a cumulative dose–response curve to cabachol was plotted. Guinea pig ileum [1] Portions of terminal ileum (2 cm) were removed at about 5 cm from the ileum–cecum junction and mounted under 1 g of tension in PSS (the same used for atria) at 37°C. Tension changes were recorded isotonically. Tissues were equilibrated for 1 h and a dose–response curve to acetylcholine was obtained. Guinea pig uterus [1] Uterine horns were divided into four portions and mounted under 1 g of tension in PSS with the following composition (mM): NaCl (154), KCl (5.63), CaCl2 (0.54), MgCl2 (0.95), NaHCO3 (5.95), glucose (2.78). The preparations were maintained at 30°C and after a 1 h equilibration period, isotonic contractions to carbachol were recorded. Initially the tissues were exposed to a single concentration of carbachol (3 μM) to check the responsiveness to the agonist, then a dose–response curve for carbachol was obtained. |
| References |
[1]. Further structure–activity relationships in the series of tropanyl esters endowed with potent antinociceptive activity. Il Farmaco 53.12 (1998): 764-772. [2].Presynaptic cholinergic modulators as potent cognition enhancers and analgesic drugs. 2. 2-Phenoxy-, 2-(phenylthio)-, and 2-(phenylamino)alkanoic acid esters. J Med Chem. 1994 May 27;37(11):1712-9. |
| Additional Infomation |
2-(4-chlorophenoxy)butanoic acid (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) ester is a monocarboxylic acid.
The frozen analogs of SM21 and SM32, which have been studied in more detail, are practically equiactive with their parent compounds, but their acute toxicity is much higher (Table 1). The same happens for the analog 16, while 12, 13, 14 are practically inactive at the dose tested. Among the analogs of 20, which have already been studied by us, only the sulfur isoster 28 is interesting, since it shows a remarkable efficacy as analgesic. We have previously shown that the antinociceptive activity of 20, despite its structural similarity with 5-HT3 antagonists, is not due to interaction with this kind of receptors but, more likely, to activation of central 5-HT4 receptors. It is possible that its isoster 28 behaves in the same way. However, due to its high toxicity, that would prevent any useful use as analgesic, we did not investigate any further its mechanism of action. In conclusion, it appears that reduction of molecular flexibility of the lead compounds maintains or slightly reduces analgesic activity but, at the same time, increases their toxicity. During our researches in this field we have noticed that toxicity somehow parallels 5-HT4 affinity. As a matter of fact, the analgesic activity of 15 at the maximal effect dose (MED=50 mg/kg) is almost completely reversed (66%) by SDZ-205557, a 5-HT4 antagonist. The reversal of the analgesic activity of SM21 was, under the same conditions, quite lower (44%). We may conclude that conformational restriction of atropine, SM21 and SM32, apparently and contrary to our expectations, favors interaction with the 5-HT4, respect to presynaptic muscarinic receptors. [1] |
Solubility Data
| Solubility (In Vitro) | Typically soluble in DMSO (e.g. 10 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2031 mL | 11.0154 mL | 22.0308 mL | |
| 5 mM | 0.4406 mL | 2.2031 mL | 4.4062 mL | |
| 10 mM | 0.2203 mL | 1.1015 mL | 2.2031 mL |