Physicochemical Properties
| Molecular Formula | C16H12F3N3S | |
| Molecular Weight | 335.35 | |
| Exact Mass | 335.07 | |
| Elemental Analysis | C, 57.31; H, 3.61; F, 17.00; N, 12.53; S, 9.56 | |
| CAS # | 305350-87-2 | |
| Related CAS # |
|
|
| PubChem CID | 9549284 | |
| Appearance | white solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 512.6±50.0 °C at 760 mmHg | |
| Melting Point | 127-128.2ºC | |
| Flash Point | 263.8±30.1 °C | |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C | |
| Index of Refraction | 1.631 | |
| LogP | 1.95 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 23 | |
| Complexity | 487 | |
| Defined Atom Stereocenter Count | 0 | |
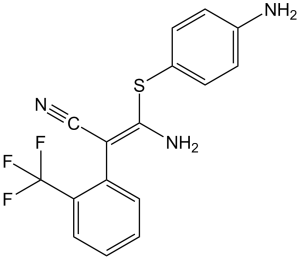
| SMILES | FC(F)(F)C1=C(/C(C#N)=C(N)/SC2=CC=C(N)C=C2)C=CC=C1 |
|
| InChi Key | JLOXTZFYJNCPIS-FYWRMAATSA-N | |
| InChi Code | InChI=1S/C16H12F3N3S/c17-16(18,19)14-4-2-1-3-12(14)13(9-20)15(22)23-11-7-5-10(21)6-8-11/h1-8H,21-22H2/b15-13+ | |
| Chemical Name | (Z)-3-amino-3-(4-aminophenyl)sulfanyl-2-[2-(trifluoromethyl)phenyl]prop-2-enenitrile | |
| Synonyms | SL 327; SL-327; SL327 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | MEK1 (IC50 = 0.18 μM); MEK2 (IC50 = 0.22 μM); AP-1 (IC50 = 2.03 μM) |
| ln Vitro | SL327 is a structural homologue of the specific MKK1/2 inhibitor U0126 with IC50 of 0.18 μM and 0.22 μM for MEK1 and MEK2 respectively. Other kinases such as PKA, PKC, or CamKII are unaffected by SL327. [1] |
| ln Vivo | SL327 (50 mg/kg) crosses the blood-brain barrierand and blocks fear conditioning by inhibiting MAPK/ERK phosphorylation. [2] SL327 (30 mg/kg) significantly reduces spatial learning in mice. [3] SL327 (50 mg/kg) inhibits the effects of cocaine. [4] |
| Enzyme Assay | Assays for protein kinases are carried out. In the beginning of each kinase assay, enzyme is added to a mixture of substrate and [γ-32P]ATP. Then, this mixture is incubated for 10 minutes at 30 or 37 degrees Celsius. By aliquoting the reaction mixture and sprinkling it on Whatman P-81 phosphocellulose filter paper, the reaction can be stopped. The papers are then dried, scintillation counted, and washed in 150 mM H3PO4. By monitoring [32P]phosphate incorporation into the substrate Kemptide (100 μM), the catalytic subunit of PKA is evaluated. The phosphorylation of the synthetic peptide Autocamtide (100 M) in the presence of 100 M Calcium and 10 μg/mL Calmodulin is used to measure the activity of CaMKII. The catalytic subunit of PKC has a preferred substrate in the form of NG(28-43) (10 μM), a synthetic peptide analog of a neurogranin fragment. Substrate phosphorylation was always a linear function of both time and enzyme concentration. |
| Animal Protocol |
Fear conditioning experiments in male Spague-Dawley rats. 10-100 mg/Kg i.p. |
| References |
[1]. J Biol Chem . 2000 Nov 24;275(47):37086-92. [2]. Nat Neurosci . 1998 Nov;1(7):602-9. [3]. Learn Mem . 1999 Sep-Oct;6(5):478-90. [4]. J Neurosci . 2000 Dec 1;20(23):8701-9. |
| Additional Infomation | SL-327 is a nitrile that is acrylonitrile in which the hydrogen attached to the same carbon as the cyano group has been replaced by an o-(trifluoromethyl)phenyl group, while the remaining hydrogens of the ethenyl group have been replaced by amino and (4-aminophenyl)sulfanyl groups. The configuration of the double bond is not specified. It is an inhibitor of MEK1 and MEK2. It has a role as an EC 2.7.12.2 (mitogen-activated protein kinase kinase) inhibitor and a neuroprotective agent. It is a member of (trifluoromethyl)benzenes, a nitrile, an enamine, an organic sulfide, a substituted aniline and a primary amino compound. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.45 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.45 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.45 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 2% DMSO+30% PEG 300+5% Tween 80+ddH2O: 5mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9820 mL | 14.9098 mL | 29.8196 mL | |
| 5 mM | 0.5964 mL | 2.9820 mL | 5.9639 mL | |
| 10 mM | 0.2982 mL | 1.4910 mL | 2.9820 mL |