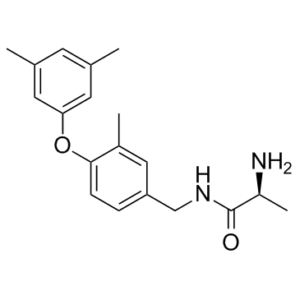
SGC2085, identified from virtual screening approaches, is a novel, potent and selective inhibitor of coactivator associated arginine methyltransferase 1 (CARM1) with an IC50 of 50 nM. Protein arginine methyltransferases (PRMTs) represent an emerging target class in oncology and other disease areas. So far, the most successful strategy to identify PRMT inhibitors has been to screen large to medium-size chemical libraries. Attempts to develop PRMT inhibitors using receptor-based computational methods have met limited success. SGC2085 which features a methyl at position R1 and a 3,5-dimethylphenoxy at R2 has an IC50 of 50 nM for CARM1 and is over 100-fold selective for CARM1 over PRMT6. These results indicate that the presence of a substituent at R1 is essential for potent and selective inhibition of CARM1. With the exception of PRMT6 (IC50=5.2 μM), SGC2085 does not inhibit other PRMTs.
Physicochemical Properties
| Molecular Formula | C19H24N2O2 | |
| Molecular Weight | 312.41 | |
| Exact Mass | 312.183 | |
| Elemental Analysis | C, 73.05; H, 7.74; N, 8.97; O, 10.24 | |
| CAS # | 1821908-48-8 | |
| Related CAS # | SGC2085 hydrochloride;1821908-49-9; 1821908-48-8 | |
| PubChem CID | 121231417 | |
| Appearance | White to off-white solid powder | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 498.3±45.0 °C at 760 mmHg | |
| Flash Point | 255.2±28.7 °C | |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C | |
| Index of Refraction | 1.568 | |
| LogP | 3.76 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 23 | |
| Complexity | 378 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | O(C1C([H])=C(C([H])([H])[H])C([H])=C(C([H])([H])[H])C=1[H])C1C([H])=C([H])C(=C([H])C=1C([H])([H])[H])C([H])([H])N([H])C([C@]([H])(C([H])([H])[H])N([H])[H])=O |
|
| InChi Key | GLFOFXKMALJTCZ-HNNXBMFYSA-N | |
| InChi Code | InChI=1S/C19H24N2O2/c1-12-7-13(2)9-17(8-12)23-18-6-5-16(10-14(18)3)11-21-19(22)15(4)20/h5-10,15H,11,20H2,1-4H3,(H,21,22)/t15-/m0/s1 | |
| Chemical Name | (2S)-2-amino-N-[[4-(3,5-dimethylphenoxy)-3-methylphenyl]methyl]propanamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CARM1 (coactivator associated arginine methyltransferase 1; IC50 = 50 nM) | ||
| ln Vitro | Twenty-one human protein methyltransferases are completely selective for SGC2085 (1 μM, 10 μM, 50 μM; 48 h) [1]. In HEK293 cells, SGC2085 (10 μM; 48 h) shows no cellular activity and minimal cell permeability [1]. | ||
| ln Vivo |
|
||
| Enzyme Assay |
Enzymatic Assays[1] A radiometric assay was used to study the in vitro inhibition of PRMTs as described previously. In principle, radiolabeled S-adenosylmethionine (3H-SAM, specific activity range 12–18 Ci/mmol) served as methyl donor, for methylation of the biotinylated histone peptides. Incorporation of the tritiated methyl into the arginine residues of the substrate histone peptides then was measured in scintillation proximity FlashPlates Plus. The amount of methylated peptides was then quantified by tracing the radioactivity (counts per minute) using TopCount NXT plate reader. The C-terminally biotinylated histone H3 peptide composed of the first 25 amino acids residues (H3 1–25) was used as substrate for CARM1. The typical assay mixture (10 μL volume) contained 25 nM CARM1, 0.7 μM H3 1–25, and 1.9 μM SAM in 20 mM bicine (pH 8.5). The IC50 values were determined under balanced conditions at apparent KM concentrations of both substrates by titration of the compound in the reaction mixture in a range between 100 and 0.006 μM. DSF[1] Differential scanning fluorimetry (DSF) measurements were performed with a Light Cycler 480 II instrument from Roche Applied Science. The protein was assayed at 0.2 mg/mL in 100 mM HEPES (pH 7.5), 150 mM NaCl, 2% DMSO final, and 5× Sypro Orange (5000× stock solution was diluted 1:1000 to yield a 5× working concentration). The compounds were titrated up to 600 μM to assess their stabilizing effect. DSF was carried out by increasing the temperature by 4 °C/min from 30 to 95 °C, and data points were collected at 0.4 °C intervals. The temperature scan curves were fitted to a Boltzmann sigmoid function, and the Tm values were obtained from the midpoint of the transition as described previously. DSLS[1] Differential static light scattering (DSLS) experiments were performed as previously described. Briefly, CARM1 at 0.2 mg/mL in 100 mM HEPES pH 7.5 and 150 mM NaCl was incubated with the titrated compound (2% DMSO final). Forty microliters of the protein/compound mixture was heated from 30 to 80 °C at a rate of 1 °C per min in a clear-bottom 384-well plate. Protein aggregation was monitored by measuring the intensity of the scattered light every 30 s with a CCD camera. These total intensities were then plotted against temperature and fitted to the Boltzman equation by nonlinear regression. SPR[1] Surface plasmon resonance (SPR) experiments were performed using a Biacore T200 at 20 °C. Approximately 4500 RU of CARM1 was amino coupled to a CM5 Chip (according to the manufacturer’s protocol), and another cell being left blank for reference subtraction. Compounds were serially diluted in DMSO and transferred to the buffer (HBS-EP) giving 5% DMSO final. Compounds were tested with 30 s contact time at 75 μL/min. KD values were determined using Steady State Affinity Fitting and the Biacore T200 Evaluation Software. SAM binding to CARM1 showed the protein to be approximately 90% functional on the chip. |
||
| Cell Assay |
Cellular Assay[1] HEK293 cells were grown in 12-well plates in DMEM supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL). Thirty percent confluent cells were treated with inhibitors or DMSO. After 48 h, media were removed and cells were lysed in 100 μL of total lysis buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 0.5% Triton X-100, 12.5 U/mL benzonase), complete EDTA-free protease inhibitor cocktail (Roche). After 3 min incubation at room temperature, SDS was added to 1% final concentration. Lysates were run on SDS-PAGE, and immunoblotting was done as outlined below to determine the levels of unmethylated and methylated BAF155. Western Blot[1] Total cell lysates were resolved in 4–12% Bis-Tris Protein Gels with MOPS buffer and transferred in for 1.5 h (80 V) onto PVDF membrane in Tris-Glycine transfer buffer containing 20% MeOH and 0.05% SDS. Blots were blocked for 1 h in blocking buffer (5% milk in 0.1% Tween 20-PBS) and incubated with primary antibodies mouse anti-BAF155 (1:500) and rabbit antidimethyl BAF155 (R1064 asymmetrically dimethylated) (1:1000) in blocking buffer overnight at 4 °C. After five washes with 0.1% Tween 20 PBS, the blots were incubated with goat-anti rabbit (IR800 conjugated) and donkey antimouse (IR 680) antibodies (1:5000) in Odyssey Blocking Buffer for 1 h at RT and washed five times with 0.1% Tween 20 PBS. The signal was read on an Odyssey scanner at 800 and 700 nm. |
||
| Animal Protocol |
|
||
| References |
[1]. Discovery of a Potent and Selective Coactivator Associated Arginine Methyltransferase 1 (CARM1) Inhibitor by Virtual Screening. J Med Chem. 2016 Jul 28;59(14):6838-47. |
||
| Additional Infomation | Protein arginine methyltransferases (PRMTs) represent an emerging target class in oncology and other disease areas. So far, the most successful strategy to identify PRMT inhibitors has been to screen large to medium-size chemical libraries. Attempts to develop PRMT inhibitors using receptor-based computational methods have met limited success. Here, using virtual screening approaches, we identify 11 CARM1 (PRMT4) inhibitors with ligand efficiencies ranging from 0.28 to 0.84. CARM1 selective hits were further validated by orthogonal methods. Two structure-based rounds of optimization produced 27 (SGC2085), a CARM1 inhibitor with an IC50 of 50 nM and more than hundred-fold selectivity over other PRMTs. These results indicate that virtual screening strategies can be successfully applied to Rossmann-fold protein methyltransferases.[1] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (8.00 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (8.00 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (8.00 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2009 mL | 16.0046 mL | 32.0092 mL | |
| 5 mM | 0.6402 mL | 3.2009 mL | 6.4018 mL | |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.2009 mL |