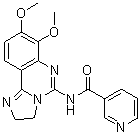
SB-242235 has the potential to treat cytokine-mediated illnesses like autoimmune or inflammatory diseases because it is a potent and selective p38 MAP kinase inhibitor with an IC50 of 1.0 uM. Positive pharmacokinetic results were seen with SB-242235. However, SB-242235 showed low to moderate clearance with plasma half-lives > 4 hours in non-rodent species. Systemic plasma clearance was high in rats. The oral bioavailability was high in all preclinical species. With increasing dose, SB-242235's clearance decreased in rats and monkeys, and at high oral doses, its apparent oral bioavailability appeared to be > 100%. These non-linear elimination kinetics were also evident in the drug's apparent oral bioavailability.
Physicochemical Properties
| Molecular Formula | C18H17N5O3 | |
| Molecular Weight | 351.36 | |
| Exact Mass | 353.165 | |
| Elemental Analysis | C, 61.53; H, 4.88; N, 19.93; O, 13.66 | |
| CAS # | 193746-75-7 | |
| Related CAS # |
|
|
| PubChem CID | 9863367 | |
| Appearance | white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 568.4±60.0 °C at 760 mmHg | |
| Flash Point | 297.5±32.9 °C | |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C | |
| Index of Refraction | 1.662 | |
| LogP | 3.01 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 26 | |
| Complexity | 442 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | FC1C([H])=C([H])C(=C([H])C=1[H])C1=C(C2C([H])=C([H])N=C(N=2)OC([H])([H])[H])N(C([H])=N1)C1([H])C([H])([H])C([H])([H])N([H])C([H])([H])C1([H])[H] |
|
| InChi Key | PDTYLGXVBIWRIM-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C19H20FN5O/c1-26-19-22-11-8-16(24-19)18-17(13-2-4-14(20)5-3-13)23-12-25(18)15-6-9-21-10-7-15/h2-5,8,11-12,15,21H,6-7,9-10H2,1H3 | |
| Chemical Name | 4-[5-(4-fluorophenyl)-3-piperidin-4-ylimidazol-4-yl]-2-methoxypyrimidine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | p38 MAPK (IC50 = 1.0 μM) |
| ln Vitro | SB 242235 (0-10 μM) inhibits MAPKAP K2 activation in a dose-dependent manner with an IC50 of 1.0 μM in human chondrocytes stimulated with IL-1β[1]. SB 242235 inhibits intracellular p38 activity, MAPKAP K2 was then isolated from these cells and assessed using HSP27 as a substrate[1]. |
| ln Vivo |
SB242235 (100 mg/kg; p.o.) abolishes MAP-KAPK-2 activity and HSP27 phosphorylation[2]. SB242235 inhibits expression of the pro-inflammatory cytokines interleukin (IL)-6 and KC (murine IL-8) and COX-2[2]. In rats and monkeys, SB-242235 has been shown to have non-linear elimination kinetics, which showed up as a decrease in clearance with dose and an apparent oral bioavailability > 100% at high oral doses[3]. |
| Enzyme Assay | SB 242235 inhibited intracellular p38 activity, human chondrocytes were treated with different doses of SB 242235 prior to stimulation with IL-1_ for 15 min. From these cells, MAPKAP K2 was then isolated and measured using HSP27 as a substrate. With an IC50 of 1.0 uM, SB 242235 inhibited the activation of MAPKAP K2 in a dose-dependent manner. |
| Animal Protocol |
Female SKH-1 hairless mice (4–6 weeks)[2] 100 mg/kg Oral administered, 30 minutes prior to ultraviolet B (UVB) irradiation |
| References |
[1]. Differential effects of SB 242235, a selective p38 mitogen-activated protein kinase inhibitor, on IL-1 treated bovine and human cartilage/chondrocyte cultures. Osteoarthritis Cartilage, 2000. 8(6): p. 434-43. [2]. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J Invest Dermatol. 2005 Jun;124(6):1318-25. [3]. SB-242235, a selective inhibitor of p38 mitogen-activated protein kinase. I: preclinical pharmacokinetics. Xenobiotica, 2002. 32(3): p. 221-33. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.07 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.07 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.07 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8461 mL | 14.2304 mL | 28.4608 mL | |
| 5 mM | 0.5692 mL | 2.8461 mL | 5.6922 mL | |
| 10 mM | 0.2846 mL | 1.4230 mL | 2.8461 mL |