Physicochemical Properties
| Molecular Formula | C24H34O6S |
| Molecular Weight | 450.59 |
| Exact Mass | 450.208 |
| Elemental Analysis | C, 63.97; H, 7.61; O, 21.30; S, 7.12 |
| CAS # | 256382-08-8 |
| PubChem CID | 9803828 |
| Appearance | Colorless to light yellow liquid |
| LogP | 2.935 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 14 |
| Heavy Atom Count | 31 |
| Complexity | 580 |
| Defined Atom Stereocenter Count | 4 |
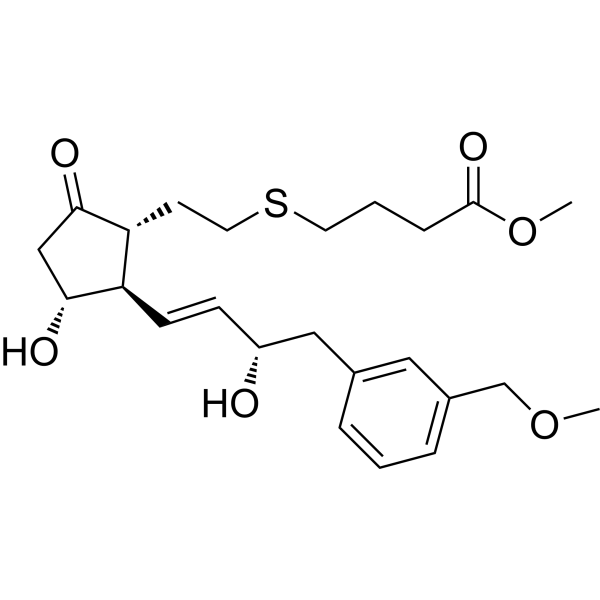
| SMILES | COCC1=CC=CC(=C1)C[C@@H](/C=C/[C@H]2[C@@H](CC(=O)[C@@H]2CCSCCCC(=O)OC)O)O |
| InChi Key | FBQUXLIJKPWCAO-AZIFJQEOSA-N |
| InChi Code | InChI=1S/C24H34O6S/c1-29-16-18-6-3-5-17(13-18)14-19(25)8-9-20-21(23(27)15-22(20)26)10-12-31-11-4-7-24(28)30-2/h3,5-6,8-9,13,19-22,25-26H,4,7,10-12,14-16H2,1-2H3/b9-8+/t19-,20-,21-,22-/m1/s1 |
| Chemical Name | methyl 4-[2-[(1R,2R,3R)-3-hydroxy-2-[(E,3S)-3-hydroxy-4-[3-(methoxymethyl)phenyl]but-1-enyl]-5-oxocyclopentyl]ethylsulfanyl]butanoate |
| Synonyms | ONO-4819; 256382-08-8; Rivenprost [INN]; ONO-4819; ONO-4819CD; UNII-1WBO45T413; methyl 4-[2-[(1R,2R,3R)-3-hydroxy-2-[(E,3S)-3-hydroxy-4-[3-(methoxymethyl)phenyl]but-1-enyl]-5-oxocyclopentyl]ethylsulfanyl]butanoate; 1WBO45T413; ONO-AE1-734 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | EP4 (Ki = 0.7 nM) |
| ln Vitro |
Rivenprost (1 nM–1 μM) stimulates osteoblast differentiation by upregulating the expression of Runx2 and Osterix, thereby increasing bone formation[1]. Rivenprost (1 nM–1 μM) inhibits adipocyte differentiation in bone by downregulating the mRNA level of PPARγ[1]. RT-PCR[1] Cell Line: C3H10T1/2 Concentration: 1 nM–1 μM Incubation Time: 7 days Result: Reduced PPARγ in a dose-dependent manner. Effects of Rivenprost (ONO-4819) on osteoblast differentiation[1] We next examined the effects of ONO-4819 on bone at the cellular level. Conventional histomorphometric analysis demonstrated that ONO-4819 significantly increased the osteoblast number in C3H10T1/2 cultures on days 3, 5, 7, and 14 (Fig. 3B). Histomorphometry using immunohistochemical staining for Runx2 and Osterix, which are essential transcription factors for osteoblast differentiation, revealed that the numbers of Runx2- and Osterix-positive osteoblasts were significantly increased as early as day 1 (Fig. 3A, B). In vitro studies showed that ONO-4819 induced ALPase activity (Fig. 3C) and increased mRNA expression of ALPase, Osterix, Col1a1, and Bglap1 in C3H10T1/2 cells. Effects of Rivenprost (ONO-4819) on adipocyte differentiation[1] Osteoblasts and adipocytes are differentiated from common progenitors in bone marrow (Bianco et al., 2001, Jiang et al., 2002), and there is an inverse relationship between their differentiation (Beresford et al., 1992). We thus examined the effects of ONO-4819 on adipocytes differentiation. Histological analysis showed that the number of adipocytes in bone marrow was increased in an age-dependent manner, which increase was significantly prevented by ONO-4819 (Fig. 4A). In vitro studies using C3H10T1/2 cells showed that ONO-4819 dose-dependently suppressed the adipocyte differentiation (Fig. 4B). Semi-quantitative RT-PCR analysis demonstrated that the mRNA expression of PPARγ, the master regulator of adipogenesis, was decreased by ONO-4819 treatment (Fig. 4C). |
| ln Vivo |
Rivenprost (10 μg/kg, injected subcutaneously for 5 weeks) increases bone formation and reduces age-dependent adipocyte levels in Sprague-Dawley rats [1]. Rivenprost (0.2 mg/kg, intraperitoneal injection, single dose) has hepatoprotective effects against GalN-/LPS-induced liver injury in Wistar rats through inflammatory cytokines such as TNF-α [2]. To explore this matter, Rivenprost (ONO-4819) (10μg/kg) was injected into intact rats twice a day for 5weeks, and their bones were then analyzed by morphological techniques. The effects of ONO-4819 on the differentiation of bone cells were also examined in vitro. Bone morphometric analysis showed that osteoblast number, bone volume, mineral apposition rate, and bone formation rate were significantly increased by ONO-4819, whereas osteoclast number was not affected. Immunohistochemical examination demonstrated that ONO-4819 increased the number of Runx2- and Osterix-positive osteoblasts in rats. [1] Researchers evaluated the efficacy of Rivenprost (ONO-4819), a newly developed agonist of a prostaglandin receptor subtype (EP4), on experimental model of acute liver injury in rats. Acute liver injury was induced by simultaneous intraperitoneal (i.p.) administration of D-galactosamine (GalN, 1 g/kg body weight) and lipopolysaccharide (LPS, 100 mg/kg body weight). The rats received a single intraperitoneal injection of Rivenprost (ONO-4819) (0.2 mg/kg body weight) or physiological saline immediately after GalN/LPS administration. Submassive hepatic necrosis with marked elevation of serum total bilirubin, serum aspartate aminotransferase and serum alanine aminotransferase levels developed 24 h after GalN/LPS administration. The administration of Rivenprost (ONO-4819) significantly inhibited the development of submassive hepatic necrosis and inhibited the elevation in levels of biochemical markers that indicate liver function. In addition, the apoptotic index of hepatocytes assessed by the TUNEL method was significantly lower in rats treated with ONO-4819 than in the control. Although serum levels of tumor necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma) and interleukin-8 (IL-8) were markedly elevated after GalN/LPS administration, ONO-4819 significantly inhibited the elevation of those of TNF-alpha and IFN-gamma but not that of IL-8. The beneficial effect of ONO-4819 for acute liver injury was similar at doses of 0.1, 0.05 and 0.01 mg/kg body weight. These results suggest that the EP4 agonist, ONO-4819, may have a protective effect against experimental liver injury in rats through the suppression of inflammatory cytokines.[2] |
| Cell Assay |
Osteoblast differentiation[1] C3H10T1/2 cells (1 × 104 cells/well) were plated in 48-well plates and cultured in α-MEM supplemented with 10% FBS and antibiotic–antimycotic in the presence or absence of Rivenprost (ONO-4819) (1 nM–1 μM) for 7 days. ALPase activity was detected as described previously (Hiraga et al., 2007). Adipocyte differentiation[1] C3H10T1/2 cells (5 × 104 cells/well) were plated in 24-well plates and cultured in α-MEM supplemented with 10% FBS, antibiotic–antimycotic, and rhBMP2 (50 ng/ml) until confluent. Then, the cells were treated with 100 nM dexamethasone, 500 μM 3-isobutyl-1-methylxanthine (IBMX), 0.1 mM indomethacin, and 10 μg/ml insulin in the presence or absence of Rivenprost (ONO-4819) (1 nM–1 μM ) for 5 days, and then made mature by a 2-day incubation in medium containing 10 μg/ml insulin in the presence or absence of Rivenprost (ONO-4819). These operations were repeated once. After fixation with 10% formaldehyde, the adipocytes were stained with Oil Red O solution and counted on 5 images, each of 0.36 mm2 (694 × 520 μm), under a light microscope. Osteoclast differentiation[1] Primary osteoblasts, isolated from calvaria of 1-day-old ddY mice by use of collagenase (Takahashi et al., 2003), were cultured for 5 days prior to use. Bone marrow cells (1.5 × 105 cells/well) from the tibiae of 6-week-old male ddY mice were co-cultured with the osteoblasts (1.5 × 105 cells/well) in 48-well plates and cultured for 7 days in α-MEM supplemented with 10% FBS in the presence or absence of Rivenprost (ONO-4819) (10 nM–10 μM). The cultures were replenished with fresh medium on day 3. At the end of the culture period, the cells were fixed with 10% formaldehyde in PBS, treated with 0.1% Triton-X in PBS, and stained for TRAPase. By light microscope observation, TRAPase-positive multinucleated cells containing more than 3 nuclei were counted as osteoclasts. Reverse transcription polymerase chain reaction (RT-PCR)[1] For Osterix mRNA expression analysis, C3H10T1/2 cells (1 x 105 cells/well) were plated in 6-well plates and cultured for 24 h. Then, the cells were treated with Rivenprost (ONO-4819) (1 nM–1 μM) for 6 h. For PPARγ mRNA, C3H10T1/2 cells (1 × 104 cells/well) were plated in 6-well plates and cultured by the method described above for 7 days. Total RNA was isolated by using a TriPure Isolation Kit (Roche Diagnostics K.K., Tokyo, Japan). The RNA was treated with DNase, after which the cDNA was synthesized with PrimeScript Reverse Transcriptase. |
| Animal Protocol |
Animal/Disease Models:Sprague Dawley rats[1] Doses: 10 μg/kg Route of Administration: s.c., twice a day for 5 weeks Experimental Results: Increased osteoblast number, bone volume, mineral apposition rate and bone formation rate. Decreased adipocyte number. Rivenprost (ONO-4819) was dissolved in saline and stored at 4 °C until use. Seven-week-old, male, Sprague–Dawley rats received Rivenprost (ONO-4819) (10 μg/kg, s.c.) twice daily for 5 weeks. The dose was chosen according to previous studies (Ito et al., 2006, Yoshida et al., 2002). Rats in the control group received saline. For bone labeling, calcein (4 mg/kg, i.p.) was injected 2 and 7 days before sacrifice. Under anesthesia with sodium pentobarbital (80 mg/kg, i.p.), rats were sacrificed and fixed by perfusion with 4% paraformaldehyde at days 1, 3, 5, 7, 14, 21, 28, and 35 after the start of agonist injections (5 animals/group at each time point). Rats were fed standard rodent chow (CRF-1) and tap water ad libitum, and maintained under conditions of 24 °C and a 12-h dark:12-h light cycle. [1] |
| References |
[1]. Prostaglandin E(2) receptor EP(4)-selective agonist (ONO-4819) increases bone formation by modulating mesenchymal cell differentiation. Eur J Pharmacol. 2011 Jan 10;650(1):396-402. [2]. , A novel prostaglandin E receptor subtype agonist, 0N0-4819, attenuates acute experimental liver injury in rats. Hepatol Res. 2001 Nov;21(3):252-260. |
| Additional Infomation |
Rivenprost is a benzyl ether. Rivenprost is under investigation in clinical trial NCT00296556 (Therapeutic Study of ONO-4819CD for Ulcerative Colitis). Prostaglandin E(2) (PGE(2)) positively regulates bone resorption and formation mainly mediated through the EP(4) receptor, a subtype of PGE(2) receptors. ONO-4819, an EP(4) receptor-selective agonist, has been shown to increase bone volume, density, and strength; however, the mechanism of these effects has yet to be fully elucidated. To explore this matter, ONO-4819 (10μg/kg) was injected into intact rats twice a day for 5weeks, and their bones were then analyzed by morphological techniques. The effects of ONO-4819 on the differentiation of bone cells were also examined in vitro. Bone morphometric analysis showed that osteoblast number, bone volume, mineral apposition rate, and bone formation rate were significantly increased by ONO-4819, whereas osteoclast number was not affected. Immunohistochemical examination demonstrated that ONO-4819 increased the number of Runx2- and Osterix-positive osteoblasts in rats. In vitro studies using the multipotent mesenchymal cell line C3H10T1/2 showed that ONO-4819 induced alkaline phosphatase (ALPase) activity and up-regulated the mRNA expression of ALPase and Osterix. In contrast, ONO-4819 reduced the mRNA expression of peroxisome proliferator-activated receptor γ (PPARγ) and inhibited adipocyte differentiation of C3H10T1/2 cells, which findings are consistent with the observation that the age-dependent increase in adipocyte number in the bone marrow was significantly suppressed in the ONO-4819-treated animals. ONO-4819 also dose-dependently increased osteoclast-like cell formation in vitro, but the required concentrations were much higher than those to induce osteoblast differentiation. These results collectively suggest that ONO-4819 increased bone formation by stimulating osteoblast differentiation and function, possibly through modulating mesenchymal cell differentiation in the bone.[1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2193 mL | 11.0966 mL | 22.1931 mL | |
| 5 mM | 0.4439 mL | 2.2193 mL | 4.4386 mL | |
| 10 mM | 0.2219 mL | 1.1097 mL | 2.2193 mL |