Physicochemical Properties
| Molecular Formula | C21H24O9 |
| Molecular Weight | 420.4099 |
| Exact Mass | 420.142 |
| Elemental Analysis | C, 66.27; H, 7.60; N, 2.58; O, 17.66; S, 5.90 |
| CAS # | 155-58-8 |
| Related CAS # | 1314795-11-3 |
| PubChem CID | 637213 |
| Appearance | Yellow to brown solid powder |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 749.3±60.0 °C at 760 mmHg |
| Melting Point | 236-240ºC |
| Flash Point | 406.9±32.9 °C |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.711 |
| LogP | 0.68 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 30 |
| Complexity | 559 |
| Defined Atom Stereocenter Count | 5 |
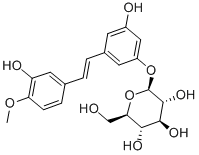
| SMILES | O1[C@]([H])([C@@]([H])([C@]([H])([C@@]([H])([C@@]1([H])C([H])([H])O[H])O[H])O[H])O[H])OC1C([H])=C(C([H])=C(/C(/[H])=C(\[H])/C2C([H])=C([H])C(=C(C=2[H])O[H])OC([H])([H])[H])C=1[H])O[H] |
| InChi Key | GKAJCVFOJGXVIA-DXKBKAGUSA-N |
| InChi Code | InChI=1S/C21H24O9/c1-28-16-5-4-11(8-15(16)24)2-3-12-6-13(23)9-14(7-12)29-21-20(27)19(26)18(25)17(10-22)30-21/h2-9,17-27H,10H2,1H3/b3-2+/t17-,18-,19+,20-,21-/m1/s1 |
| Chemical Name | (2S,3R,4S,5S,6R)-2-[3-hydroxy-5-[(E)-2-(3-hydroxy-4-methoxyphenyl)ethenyl]phenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
| Synonyms | Radalbuvir; GS-9669; GS9669; GS 9669 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| References |
[1]. Induction of apoptosis by rhapontin having stilbene moiety, a component of rhubarb (Rheum officinale Baillon) in human stomach cancer KATO III cells. Oncol Rep. 2007 Aug;18(2):347-51. |
| Additional Infomation |
Trans-rhaponticin is a rhaponticin in which the double bond adopts a trans-configuration. It possesses a range of pharmacological activities including antitumour, antiinflammatory, antilipemic and neuroprotective activities. It has a role as an anti-inflammatory agent, a plant metabolite, a neuroprotective agent, an EC 2.3.1.85 (fatty acid synthase) inhibitor, an antineoplastic agent, an apoptosis inducer, an angiogenesis inhibitor, a hypoglycemic agent, an anti-allergic agent and an antilipemic drug. Rhapontin has been reported in Rheum likiangense, Rheum franzenbachii, and other organisms with data available. |
Solubility Data
| Solubility (In Vitro) | DMSO: 84~250 mg/mL (199.8~594.7 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.95 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.95 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3786 mL | 11.8932 mL | 23.7863 mL | |
| 5 mM | 0.4757 mL | 2.3786 mL | 4.7573 mL | |
| 10 mM | 0.2379 mL | 1.1893 mL | 2.3786 mL |