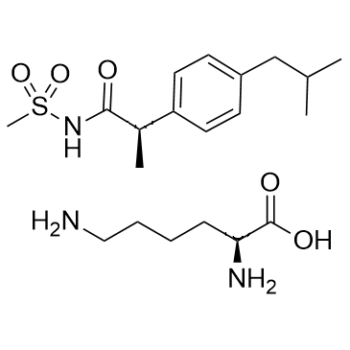
Reparixin L-lysine salt, the L-lysine salt form of reparixin, is a novel, potent small molecule weight allosteric inhibitor of chemokine receptor 1/2 (CXCR1/2) activation. It is the first medication candidate that is presently being studied in a clinical setting to prevent organ transplant recipients from suffering from ischemia/reperfusion injury. A computer-aided design program for dual allosteric CXCR1 and CXCR2 inhibitors has been developed using the binding mode of reparixin to CXCR1. Repertaxin and CXCR1 interact through a noncompetitive allosteric mode that locks CXCR1 in an inactive conformation to stop signaling, according to structural and biochemical data. In vivo, repertaxin effectively inhibits the recruitment of polymorphonuclear cells and shields organs from reperfusion injury. An overall tactic to control the activity of chemoattractant receptors is to target the Repertaxin interaction site of CXCR1.
Physicochemical Properties
| Molecular Formula | C₂₀H₃₅N₃O₅S | |
| Molecular Weight | 429.57 | |
| Exact Mass | 429.23 | |
| Elemental Analysis | C, 53.97; H, 8.03; N, 8.58; O, 22.87; S, 6.55 | |
| CAS # | 266359-93-7 | |
| Related CAS # | Reparixin; 266359-83-5 | |
| PubChem CID | 9932389 | |
| Appearance | Off-white to light yellow solid powder | |
| LogP | 4.913 | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 10 | |
| Heavy Atom Count | 29 | |
| Complexity | 495 | |
| Defined Atom Stereocenter Count | 2 | |
| SMILES | S(C([H])([H])[H])(N([H])C([C@]([H])(C([H])([H])[H])C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])=O)(=O)=O.O([H])C([C@]([H])(C([H])([H])C([H])([H])C([H])([H])C([H])([H])N([H])[H])N([H])[H])=O |
|
| InChi Key | JEJFWWFZAQBZMJ-GVKMLHTLSA-N | |
| InChi Code | InChI=1S/C14H21NO3S.C6H14N2O2/c1-10(2)9-12-5-7-13(8-6-12)11(3)14(16)15-19(4,17)18;7-4-2-1-3-5(8)6(9)10/h5-8,10-11H,9H2,1-4H3,(H,15,16);5H,1-4,7-8H2,(H,9,10)/t11-;5-/m10/s1 | |
| Chemical Name | (2S)-2,6-diaminohexanoic acid;(2R)-2-[4-(2-methylpropyl)phenyl]-N-methylsulfonylpropanamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CXCR1 ( IC50 = 1 nM ); CXCR2 ( IC50 ∼ 100 nM ); CXCR1Ile43Val ( IC50 = 80 nM ); CXCR1wtwt ( IC50 = 5.6 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | Reparixin L-lysine salt is a new and powerful small molecular weight allosteric inhibitor of chemokine receptor 1/2 (CXCR1/2) activation. It is the L-lysine salt form of reparixin. Reparixin, as demonstrated in particular experiments on CXCR1/L1.2 and CXCR2/L1.2 transfected cells and on human PMNs, is a strong functional inhibitor of CXCL8-induced biological activities on human PMNs with a marked selectivity (about 400-fold) for CXCR1. Reparixin's effectiveness is considerably reduced in L1.2 cells that express the CXCR1 Ile43Val mutant (IC50 values for CXCR1 wt and CXCR1 Ile43Val, respectively, are 5.6 nM and 80 nM). | |
| Cell Assay | L1.2 Cell suspension (1.5-3×106 cells/mL) is then seeded in triplicate in the upper compartment of the chemotactic chamber after being incubated for 15 min at 37°C with either vehicle or Reparixin (1 nM-1μM). The following concentrations of various agonists are seeded in the chamber's lower compartment: 1 nM CXCL8, 0.03 nM fMLP, 10 nM CXCL1, 2.5 nM CCL2, and 30 nM C5a. The chemotactic chamber is incubated for 45 minutes (human PMNs) or 2 hours (monocytes) at 37°C in air with 5% CO2. After the incubation period, the filter is taken out, cleaned, and stained. Five oil immersion fields are counted for each migration at a high magnification of 100×, following sample coding. Transwell filters with a pore size of 5 μm are used to assess L1.2 migration. | |
| Animal Protocol |
|
|
| References |
[1]. Design of noncompetitive interleukin-8 inhibitors acting on CXCR1 and CXCR2. J Med Chem. 2007 Aug 23;50(17):3984-4002. [2]. Receptor binding mode and pharmacological characterization of a potent and selective dual CXCR1/CXCR2non-competitive allosteric inhibitor. Br J Pharmacol. 2012 Jan;165(2):436-54. [3]. Species differences in the pharmacokinetics and metabolism of reparixin in rat and dog. Xenobiotica. 2006 May;36(5):419-40. [4]. METHODS AND COMPOUNDS FOR THE TREATMENT OF BONE LOSS AND/OR PAIN. US 20170105971 A1. [5]. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11791-6. |
|
| Additional Infomation |
Reparixin is a monoterpenoid. Reparixin has been used in trials studying the treatment and prevention of Breast Cancer, Metastatic Breast Cancer, Pancreatectomy for Chronic Pancreatitis, Islet Transplantation in Diabetes Mellitus Type 1, and Pancreatic Islet Transplantation in Type 1 Diabetes Mellitus. Reparixin is an orally available inhibitor of CXC chemokine receptor types 1 (CXCR1) and 2 (CXCR2), with potential antineoplastic activity. Upon administration, reparixin allosterically binds to CXCR1 and prevents CXCR1 activation by its ligand interleukin 8 (IL-8 or CXCL8). This may cause cancer stem cell (CSC) apoptosis and may inhibit tumor cell progression and metastasis. CXCR1, overexpressed on CSCs, plays a key role in CSC survival and the ability of CSC to self-renew; it is also linked to tumor resistance to chemotherapy. Inhibition of the IL-8/CXCR1 interaction also potentiates the cytotoxic effect of chemotherapeutic agents. In addition, reparixin inhibits CXCR2 activation and may reduce both neutrophil recruitment and vascular permeability during inflammation or injury. Drug Indication Treatment of Coronavirus disease 2019 (COVID-19) Treatment of coronavirus disease 2019 (COVID-2019) Prevention of graft rejection The chemokine CXC ligand 8 (CXCL8)/IL-8 and related agonists recruit and activate polymorphonuclear cells by binding the CXC chemokine receptor 1 (CXCR1) and CXCR2. Here we characterize the unique mode of action of a small-molecule inhibitor (Repertaxin) of CXCR1 and CXCR2. Structural and biochemical data are consistent with a noncompetitive allosteric mode of interaction between CXCR1 and Repertaxin, which, by locking CXCR1 in an inactive conformation, prevents signaling. Repertaxin is an effective inhibitor of polymorphonuclear cell recruitment in vivo and protects organs against reperfusion injury. Targeting the Repertaxin interaction site of CXCR1 represents a general strategy to modulate the activity of chemoattractant receptors.[3] Background and purpose: Acute lung injury (ALI) remains a major challenge in critical care medicine. Both neutrophils and chemokines have been proposed as key components in the development of ALI. The main chemokine receptor on neutrophils is CXCR2, which regulates neutrophil recruitment and vascular permeability, but no small molecule CXCR2 inhibitor has been demonstrated to be effective in ALI or animal models of ALI. To investigate the functional relevance of the CXCR2 inhibitor Reparixin in vivo, we determined its effects in two models of ALI, induced by either lipopolysaccharide (LPS) inhalation or acid instillation. Experimental approach: In two ALI models in mice, we measured vascular permeability by Evans blue and evaluated neutrophil recruitment into the lung vasculature, interstitium and airspace by flow cytometry. [2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.82 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.82 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.82 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 40 mg/mL (93.12 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3279 mL | 11.6395 mL | 23.2791 mL | |
| 5 mM | 0.4656 mL | 2.3279 mL | 4.6558 mL | |
| 10 mM | 0.2328 mL | 1.1640 mL | 2.3279 mL |