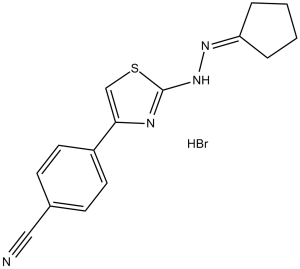
Remodelin HBr, the hydrobromide salt of Remodelin and a cell-permeable and stable analog of CPTH2, is an acetyl-transferase NAT10 inhibitor. Remodelin inhibits acetyl-transferase NAT10, a nuclear N-acetyltransferase involved in stabilization of microtubules. Remodelin was found to correct cell defects associated with progeria, restoring and improving nuclear shape and reducing the DNA damage believed to be associated with mutations in the gene for laminin A. Remodelin can improve nuclear architecture, chromatin organization, and fitness of both human lamin A/C-depleted cells and HGPS-derived patient cells, and decrease markers of DNA damage in these cells. Remodelin is a useful chemical tool to study how NAT10 affects nuclear architecture and suggest alternative strategies for treating laminopathies and aging.
Physicochemical Properties
| Molecular Formula | C15H15BRN4S | |
| Molecular Weight | 363.28 | |
| Exact Mass | 362.02 | |
| Elemental Analysis | C, 49.59; H, 4.16; Br, 22.00; N, 15.42; S, 8.83 | |
| CAS # | 1622921-15-6 | |
| Related CAS # | Remodelin;949912-58-7 | |
| PubChem CID | 86280479 | |
| Appearance | Light yellow to yellow solid | |
| LogP | 5.054 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 21 | |
| Complexity | 397 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | Br[H].S1C([H])=C(C2C([H])=C([H])C(C#N)=C([H])C=2[H])N=C1N([H])/N=C1/C([H])([H])C([H])([H])C([H])([H])C/1([H])[H] |
|
| InChi Key | XNWBCMSPDCSWSD-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C15H14N4S.BrH/c16-9-11-5-7-12(8-6-11)14-10-20-15(17-14)19-18-13-3-1-2-4-13;/h5-8,10H,1-4H2,(H,17,19);1H | |
| Chemical Name | 4-[2-(2-Cyclopentylidenehydrazinyl)-4-thiazolyl]benzonitrile Hydrobromide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | NAT10/N-acetyltransferase 10 |
| ln Vitro | Remodelin hydrobromide (10-40 μM, 1-7 days) suppresses NAT10 activity and cell proliferation in a dose-dependent manner in AR-positive and AR-negative prostate cancer cells [2]. Remodelin hydrobromide (20 μM, 24 hours) inhibits NAT10 and decreases DNA replication in prostate cancer cells [2]. In LmnaG609G/G609G fibroblasts, remodelin hydrobromide (1 μM, 7 days) decreases nuclear morphological defects and promotes genome stability [3]. |
| ln Vivo | Remodelin hydrobromide (2 or 20 mg/kg, intraperitoneally, once every two days for 4 weeks) effectively decreases the growth of AR-negative prostate cancer in a tumor xenograft nude mice model [2]. Remodelin hydrobromide (100 mg/kg, oral) inhibits NAT10 and dramatically increases healthy lifespan in the LmnaG609G/G609G mouse premature aging syndrome (HGPS) model [3]. Remodelin hydrobromide (5 mg/kg, oral) revealed a T1/2 of 1.81 hours and an oral bioavailability (F%) of 43.5% in mice [3]. Pharmacokinetic characteristics for Remodelin hydrobromide in Mice[1] Route Dose (mg/kg) T1/2 (h) Tmax (h) Cmax (ng/mL) AUC0-t (ng/h/mL) AUC0-Ꝏ (ng/h /mL) MRT_last (h) F(%) po 5 1.81 0.25 409 235 259 0.84 43.5% |
| Enzyme Assay |
Lysine acetyltransferase (KAT) assay.[1] The KAT assay was performed using the Fluorescent HAT Assay kit using NAT10 purified from HEK293 cells and 5 μg of purified MAP enriched Tubulin Porcine as a substrate. Remodelin and clickable molecule 2 were used at 50 μM. [1] Circular Dichroism Spectroscopy.[1] CD experiments were performed using a Chirascan Circular Dichroism Spectrophotometer. 200 μl of purified FLAG-NAT10 at a final concentration of 10 μM in TBS + 0.1 % NP-40 was placed in a quartz cuvette with an optical path length of 1 mm, transferred to the spectrophotometer. CD scans were recorded at 25°C over the wavelength range of 180 to 350 nm with a 1s response time, 1 nm pitch and 1.5-nm bandwidth. Compound 1 solubilized in DMSO was added and the solution was incubated for 5 min before recording scans. CD spectra were buffer subtracted, zero corrected at 300 nm and normalized (Molar ellipticity θ is quoted in 105 deg cm2 dmol−1). |
| Cell Assay |
Cell Proliferation Assay[2] Cell Types: Prostate cancer cell lines VCaP, PC3, and DU145 Tested Concentrations: 0,10,20,40 μM Incubation Duration: 1,2,7 days Experimental Results: Inhibited NAT10 and suppressed the growth of both AR- positive and AR-negative prostate cancer cells. Displayed Dramatically diminished cell proliferation activity over time, compared to the control group. diminished colony formation ability with a dose-dependent manner. Immunofluorescence[2] Cell Types: Prostate cancer cell lines VCaP, PC3, and DU145 Tested Concentrations: 20 μM Incubation Duration: 24 h Experimental Results: demonstrated a significant decrease in both the positive labeling rate and the fluorescence intensity compared to the control group. Dramatically decreased both the staining foci of IdU and the staining foci of CldU compared to control group. Western Blot Analysis[3] Cell Types: Skin fibroblasts from LmnaG609G/G609G and wildtype (WT) littermates Tested Concentrations: 1 μM Incubation Duration: 7 days Experimental Results: diminished the higher level of the DNA double-strand b |
| Animal Protocol |
Animal/Disease Models: PC-3 cells tumor xenograft model in nude athymic BALB/c nu/nu (nude) mice[2] Doses: 2 or 20 mg /kg Route of Administration: intraperitoneal (ip)injection, once every two days for 4 weeks Experimental Results: Dramatically decreased AR-negative prostate cancer tumor growth. In the high-dose group, xenograft tumor weight at the endpoint was much smaller than that in the low -dose group. Animal/Disease Models: LmnaG609G/G609G hutchinson-gilford progeria syndrome (HGPS) mouse model[3] Doses: 100 mg/kg Route of Administration: po (oral gavage), daily schedule for 3 weeks of age onward, until the end- point Experimental Results: Ameliorated age-dependent weight loss. Ameliorated cardiac pathology. Led to the dramatic amelioration of HGPS cardiac pathologies, including reduction of adventitial fibrosis of the aorta, rescue of vascular smooth muscle cell loss, and salvage of smooth muscle actin (SMA) loss, both in the aorta and the coronary arteries. Animal/Disease Models: WT Mice (pharmacokinetic/PK assay)[3] Doses: 5 mg/kg Route of Administration: Oral gav |
| References |
[1]. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science. 2014 May 2;344(6183):527-32. [2]. Ma N, et.al. Inhibition of N-Acetyltransferase 10 Suppresses the Progression of Prostate Cancer through Regulation of DNA Replication. Int J Mol Sci. 2022 Jun 12;23(12):6573. [3]. Balmus G, et.al. Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome. Nat Commun. 2018 Apr 27;9(1):1700. [4]. Zhang X, et.al. N-Acetyltransferase 10 Enhances Doxorubicin Resistance in Human Hepatocellular Carcinoma Cell Lines by Promoting the Epithelial-to-Mesenchymal Transition. Oxid Med Cell Longev. 2019 Jul 1;2019:7561879. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 10 mg/mL (27.53 mM) in 15% Cremophor EL + 85% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.88 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.88 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 4: ≥ 2.5 mg/mL (6.88 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7527 mL | 13.7635 mL | 27.5270 mL | |
| 5 mM | 0.5505 mL | 2.7527 mL | 5.5054 mL | |
| 10 mM | 0.2753 mL | 1.3763 mL | 2.7527 mL |