|
RS-127445 (RS 127445; RS127445) is a novel, potent, selective, and orally bioavailable 5-HT2B receptor antagonist with important biological activity. It can inhibit 5-HT2B and has >1000-fold selectivity for 5-HT2B compared to other 5-HT receptors with a pKi of 9.5 and pIC50 of 10.4.
|
Physicochemical Properties
| Molecular Formula |
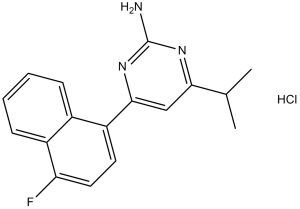
C17H16FN3
|
| Molecular Weight |
281.33
|
| Exact Mass |
317.109
|
| Elemental Analysis |
C, 72.58; H, 5.73; F, 6.75; N, 14.94
|
| CAS # |
199864-87-4
|
| Related CAS # |
RS-127445 hydrochloride; 199864-86-3
|
| PubChem CID |
196968
|
| Appearance |
Solid powder
|
| Density |
1.213g/cm3
|
| Boiling Point |
472.8ºC at 760 mmHg
|
| Flash Point |
239.8ºC
|
| Vapour Pressure |
4.13E-09mmHg at 25°C
|
| Index of Refraction |
1.637
|
| LogP |
5.524
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
2
|
| Heavy Atom Count |
21
|
| Complexity |
349
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
C1(N)=NC(C(C)C)=CC(C2=C3C(C=CC=C3)=C(F)C=C2)=N1
|
| InChi Key |
ZZZQXCUPAJFVBN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21)
|
| Chemical Name |
4-(4-fluoronaphthalen-1-yl)-6-propan-2-ylpyrimidin-2-amine
|
| Synonyms |
| MT500; RS-127445; RS 127445; RS127445; MT-500; MT 500 |
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder-20°C 3 years
4°C 2 years
In solvent -80°C 6 months
-20°C 1 month
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
|
Biological Activity
| Targets |
5-HT2B ( pIC50 = 10.4 ); 5-HT2B ( pIC50 = 9.5 )
|
| ln Vitro |
In vitro activity: RS-127445 is discovered to have 1,000 fold selectivity for the 5-HT2B receptor and nanomolar affinity (pKi=9.5±0.1) for this receptor when compared to many other receptor and ion channel binding sites. When expressed in CHO-K1 cells, human recombinant 5-HT2B receptors exhibit a potent displacement of [3H]-5-HT by RS-127445. 9.5±0.1 (n=9) is the affinity (pKi value) of RS-127445 for the 5-HT2B receptor. With a roughly 1000-fold lower affinity for the human recombinant 5-HT2A, 5-HT2C, 5-HT5, 5-HT6, and 5-HT7 receptors, a 5-HT1A receptor in rat brain membranes, a 5-HT1B/D receptor in bovine caudate, and a monoamine uptake site in rabbit platelets, RS-127445 is selective for the 5-HT2B receptor. In HEK-293 cells expressing the 5-HT2B receptor, RS-127445 potently inhibits the increases in intracellular calcium concentrations evoked by 5-HT (10 nM) (pIC50 of 10.4±0.1). RS-127445 potently inhibits 5-HT-evoked formation of inositol phosphates (pKB=9.5±0.1) and intracellular calcium increases (pIC10=10.4±0.1) in cells expressing human recombinant 5-HT2B receptors. Additionally, RS-127445 inhibits (±)α-methyl-5-HT-mediated relaxation of the rat jugular vein (pA2=9.9±0.3) as well as 5-HT-evoked contraction of the rat isolated stomach fundus (pA2B=9.5±1.1)[1]. |
|
| ln Vivo |
| The fraction of RS-127445 that is bioavailable in rats through the intraperitoneal or oral routes is 60% and 14%, respectively. RS-127445 (5 mg/kg) administered intraperitoneally resulted in plasma concentrations that were expected to completely saturate accessible 5-HT2B receptors for a minimum of 4 hours. Rats are given oral, intraperitoneal, and intravenous injections of RS-127445 (5 mg/kg). Peak plasma concentrations are reached quickly, and the highest concentrations are found by 0.25 hours after oral dosing and at the first time-point measured after intravenous and intraperitoneal administration (0.08 h). With an approximate terminal elimination half-life of 1.7 hours, RS-127445 is eliminated from plasma. When RS-127445 is administered orally or intraperitoneally, its bioavailability is roughly 14% and 62%, respectively, of what it is when administered intravenously. Of RS-127445 (5 mg/kg), about 60% of the intraperitoneal dose and 14% of the oral dose are bioavailable[1]. Faecal output is dose-dependently reduced by RS-127445 (1–30 mg/kg), with a significant reduction observed at 10 and 30 mg/kg (n=6–11). More than 98% of RS-127445 is protein-bound in the blood and brain[2]. |
|
| Enzyme Assay |
Testing the compound's affinity at more than 100 additional ion channel or receptor binding sites allows researchers to determine how selective RS-127445 is for 5-HT2B receptors. 2 mM EDTA in phosphate buffered saline is used to harvest CHO-K1 cells expressing human 5-HT2A, 5-HT2B, or 5-HT2C receptors. The process involves four rounds of centrifugation (48,000×g for 15 min) and homogenization to prepare cell membranes. The purpose of every assay is to optimize specific binding and reach steady state conditions. The 5-HT2A receptor is detected by incubating membranes from 1×106 cells with 0.2 nM [3 H]-ketanserin for 60 minutes at 32 °C. We use 10 μM methysergide to measure nonspecific binding. 0.2 nM [3 H]-5-HT is incubated for 120 minutes at 48 °C on membranes from 1.5×106 cells in order to detect the 5-HT2B receptor. 10-fold increase in 5-HT is used to measure nonspecific binding. Membranes from 3x10^5 cells are incubated with 0.5 nM [3 H]-mesuler-gine at 32 °C for 60 minutes in order to detect the 5-HT2Creceptor. Ten micrograms of methysergide are used to measure nonspecific binding. Glass fiber filters (GF/B) that have been pretreated with 0.1% polyethylene imine are used to vacuum filter out samples to end assays. Liquid scintillation counting is used to calculate bound and total radioactivity. In all of these tests, specific binding of greater than 90% is attained.
|
| Cell Assay |
For 20 minutes at 37°C, 240 μl of HEK-293 cells expressing the human 5-HT2B receptor suspension are pre-incubated with RS-127445, vehicle, or other antagonists. HEK-293 cells are cultured in 162 cm2 flasks with [3H]-myoinositol (1.67 μCi/ml) in an inositol-free Hams F12 medium containing 10% dialyzed foetal bovine serum for an entire night at 37 °C. After being harvested, the cells are resuspended at a density of roughly 3×103 cells/ml in inositol-free Hams F12 media after being washed five times with phosphate buffered saline. The addition of 5-HT starts the reactions. After the reactions have lasted for sixty minutes, 50 μl of ice-cold 20% perchloric acid is added, the mixture is chilled in an ice-water bath for ten minutes, and 160 μl of 1 N KOH is neutralized. Two milliliters of room-temperature, 50 mM Tris-HCl (pH 7.4) are used to dilute each sample. After being cleaned with 5 ml of distilled water, the aqueous portion (2.2 ml) is transferred onto Dowex AG1X8 columns (1 ml, 1: 1, w/v). Following an 18 ml distilled water wash, 3 ml of 1 N HCl is used to elute the inositol phosphates from the columns. Using a Packard 1900CA analyzer, liquid scintillation spectroscopy is used to measure the eluted radioactivity.
|
| Animal Protocol |
| Dissolved in ethanol:pro-pylene glycol: water (10: 50: 40, v/v/v); 5 mg/kg; Oral for 2.5 h ,intraperitoneal and intravenousroutes for 0.08 h | | Rats | |
|
| References |
[1]. RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist. Br J Pharmacol. 1999 Jul;127(5):1075-82.
|
|
Solubility Data
| Solubility (In Vitro) |
| DMSO: ~56 mg/mL (~199.1 mM) | | Water: <1 mg/mL | | Ethanol: ~8 mg/mL (~28.4 mM) |
|
| Solubility (In Vivo) |
5%DMSO + Corn oil: 2.8mg/ml (9.95mM) (Please use freshly prepared in vivo formulations for optimal results.)
|
| Preparing Stock Solutions |
|
1 mg |
5 mg |
10 mg |
| 1 mM |
3.5545 mL |
17.7727 mL |
35.5454 mL |
| 5 mM |
0.7109 mL |
3.5545 mL |
7.1091 mL |
| 10 mM |
0.3555 mL |
1.7773 mL |
3.5545 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles. |