RO 46-8443 is a novel, potent, selective and non-peptidic antagonist of the endothelin ETB receptor. RO 46-8443 demonstrates a selectivity of up to 2000 times for ETB receptors in terms of both functional inhibition and binding inhibitory potency. Ro 46-8443 reduced blood pressure in normotensive rats, but it created a pressor effect in SHR and DOCA rats because it blocked the ETB-mediated release of nitric oxide, which was inhibited by L-NAME. In rats treated with chronic L-NAME to hypertension, Ro 46-8443 had a depressor effect rather than a pressor effect. Therefore, Ro 46-8443 indicates that endothelial "vasorelaxant" ETB receptors have a predominant influence in DOCA rats and SHR, whereas in normotensive rats, ETB receptors appear to have a predominant role in mediating a vasoconstrictor tone.
Physicochemical Properties
| Molecular Formula | C31H35N3O8S | |
| Molecular Weight | 609.69 | |
| Exact Mass | 609.214 | |
| Elemental Analysis | C, 61.07; H, 5.79; N, 6.89; O, 20.99; S, 5.26 | |
| CAS # | 175556-12-4 | |
| Related CAS # |
|
|
| PubChem CID | 5312146 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 683.1±65.0 °C at 760 mmHg | |
| Flash Point | 367.0±34.3 °C | |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C | |
| Index of Refraction | 1.602 | |
| LogP | 3.22 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 11 | |
| Rotatable Bond Count | 13 | |
| Heavy Atom Count | 43 | |
| Complexity | 928 | |
| Defined Atom Stereocenter Count | 1 | |
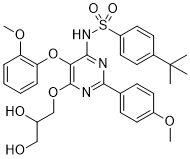
| SMILES | S(C1C([H])=C([H])C(=C([H])C=1[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])(N([H])C1=C(C(=NC(C2C([H])=C([H])C(=C([H])C=2[H])OC([H])([H])[H])=N1)OC([H])([H])C([H])(C([H])([H])O[H])O[H])OC1=C([H])C([H])=C([H])C([H])=C1OC([H])([H])[H])(=O)=O |
|
| InChi Key | DRIHNVYRUGBDHI-JOCHJYFZSA-N | |
| InChi Code | InChI=1S/C31H35N3O8S/c1-31(2,3)21-12-16-24(17-13-21)43(37,38)34-29-27(42-26-9-7-6-8-25(26)40-5)30(41-19-22(36)18-35)33-28(32-29)20-10-14-23(39-4)15-11-20/h6-17,22,35-36H,18-19H2,1-5H3,(H,32,33,34)/t22-/m1/s1 | |
| Chemical Name | 4-tert-butyl-N-[6-[(2R)-2,3-dihydroxypropoxy]-5-(2-methoxyphenoxy)-2-(4-methoxyphenyl)pyrimidin-4-yl]benzenesulfonamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | ETB ( IC50 = 34-69 nM ); ETA ( IC50 = 6800 nM ) |
| References |
[1]. In vitro characterisation of Ro 46-8443, the first non-peptide antagonist selective for the endothelin ETB receptor. FEBS Lett. 1996;383(1-2):37-41. [2]. The role of ETB receptors in normotensive and hypertensive rats as revealed by the non-peptide selective ETB receptor antagonist Ro 46-8443. FEBS Lett. 1996;383(1-2):42-45. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.41 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.08 mg/mL (3.41 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.41 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6402 mL | 8.2009 mL | 16.4018 mL | |
| 5 mM | 0.3280 mL | 1.6402 mL | 3.2804 mL | |
| 10 mM | 0.1640 mL | 0.8201 mL | 1.6402 mL |