Pyridoxylamine, one form of vitamin B₆, is used as a dietary supplement, often as the hydrochloride salt, pyridoxamine dihydrochloride. However, in the United States, the FDA ruled in January 2009 that pyridoxamine must be regulated as a pharmaceutical drug because it is the active ingredient in Pyridorin, a drug designed by Biostratum, Inc., to prevent the progression of diabetic nephropathyis. It is also an advanced glycation end production (AGEs) and lipoxidation end products (ALEs) inhibitor, to protect against diabetes-induced retinal vascular lesions.
Physicochemical Properties
| Molecular Formula | C₈H₁₂N₂O₂ |
| Molecular Weight | 168.19 |
| Exact Mass | 168.09 |
| CAS # | 85-87-0 |
| PubChem CID | 1052 |
| Appearance | White to yellow solid powder |
| Density | 1.282g/cm3 |
| Boiling Point | 460.1ºC at 760 mmHg |
| Flash Point | 232.1ºC |
| Index of Refraction | 1.617 |
| LogP | 0.746 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 12 |
| Complexity | 143 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | NHZMQXZHNVQTQA-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C8H12N2O2/c1-5-8(12)7(2-9)6(4-11)3-10-5/h3,11-12H,2,4,9H2,1H3 |
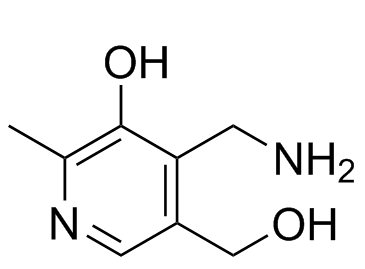
| Chemical Name | 4-(aminomethyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Pyridoxylamine (PM), a B6 vitamer, is an effective scavenger of reactive carbonyls, preventing the late stages of glycation processes that result in AGE formation[1]. |
| ln Vivo | Pyridoxylamine finally prevents the development of nephropathy in STZ-diabetic rats by limiting the production of CML and CEL as well as cross-linking in skin collagen. Since pyridoxylamine does not stop lipid peroxidation events, it does not seem to have an antioxidant effect. In addition, it inhibits the development of 4-hydroxynonenal adducts and malondialdehyde on protein in Zucker rats in vivo, which are results of lipid peroxidation that modify proteins[1]. |
| References |
[1]. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002 Sep;51(9):2826-32. |
| Additional Infomation |
Pyridoxamine is a monohydroxypyridine that is pyridine substituted by a hydroxy group at position 3, an aminomethyl group at position 4, a hydroxymethyl group at position 5 and a methyl group at position 2. The 4-aminomethyl form of vitamin B6, it is used (in the form of the hydrochloride salt) for treatment of diabetic nephropathy. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a plant metabolite, a mouse metabolite, an iron chelator and a nephroprotective agent. It is a hydroxymethylpyridine, a monohydroxypyridine, an aminoalkylpyridine and a vitamin B6. It is a conjugate base of a pyridoxaminium(1+). Pyridoxamine has been used in trials studying the treatment of Kidney Stones. Pyridoxamine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Pyridoxamine has been reported in Glycine max, Drosophila melanogaster, and other organisms with data available. The 4-aminomethyl form of VITAMIN B 6. During transamination of amino acids, PYRIDOXAL PHOSPHATE is transiently converted into pyridoxamine phosphate. |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~16.67 mg/mL (~99.11 mM) H2O : ~6.25 mg/mL (~37.16 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (12.37 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (12.37 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (12.37 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.9457 mL | 29.7283 mL | 59.4566 mL | |
| 5 mM | 1.1891 mL | 5.9457 mL | 11.8913 mL | |
| 10 mM | 0.5946 mL | 2.9728 mL | 5.9457 mL |