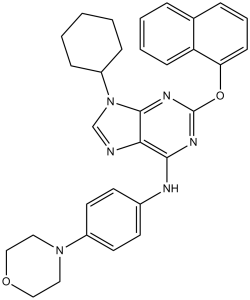
Purmorphamine (also called Shh Signaling Antagonist VI) is a potent purine-based agonist of the hedgehog signaling pathway-smoothened receptor with an EC50 of 1 μM. Given the significance of the Hedgehog (Hh) signaling pathway in regulating embryonic patterning, tissue regeneration, stem cell renewal, and cancer growth, it may find application as a therapeutic agent for bone diseases such as osteoporosis.
Physicochemical Properties
| Molecular Formula | C31H32N6O2 | |
| Molecular Weight | 520.62 | |
| Exact Mass | 520.258 | |
| Elemental Analysis | C, 71.52; H, 6.20; N, 16.14; O, 6.15 | |
| CAS # | 483367-10-8 | |
| Related CAS # | Purmorphamine; 483367-10-8 | |
| PubChem CID | 5284329 | |
| Appearance | Solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 790.3±70.0 °C at 760 mmHg | |
| Melting Point | 210-212ºC | |
| Flash Point | 431.8±35.7 °C | |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C | |
| Index of Refraction | 1.711 | |
| LogP | 4.52 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 39 | |
| Complexity | 768 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | N1(C2=CC=C(NC3=NC(OC4=CC=CC5=C4C=CC=C5)=NC6=C3N=CN6C7CCCCC7)C=C2)CCOCC1 |
|
| InChi Key | FYBHCRQFSFYWPY-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C31H32N6O2/c1-2-9-25(10-3-1)37-21-32-28-29(33-23-13-15-24(16-14-23)36-17-19-38-20-18-36)34-31(35-30(28)37)39-27-12-6-8-22-7-4-5-11-26(22)27/h4-8,11-16,21,25H,1-3,9-10,17-20H2,(H,33,34,35) | |
| Chemical Name | 9-cyclohexyl-N-(4-morpholin-4-ylphenyl)-2-naphthalen-1-yloxypurin-6-amine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Smoothened ( IC50 = 1.5 μM ) | |
| ln Vitro |
Purmorphamine directly binds to and activates Smoothened, competing with cyclopamine, an antagonist of Smo, to activate the Hedgehog pathway with an IC50 of approximately 1.5 μM.[1] Purmorphamine stimulates osteogenesis in multipotent C3H10T1/2 cells in a powerful way. In C3H10T1/2 cells, the EC50 for purmorphamine is 1 μM, as determined by ALP expression. In 3T3-L1 cells, the combination of purmorphamine (1 μM) and BMP-4 (100 ng/mL) increases ALP activity by over 90 times.[2] Purmorphamine, as opposed to BMP-4, causes osteogenesis in multipotent mesenchymal progenitor cells by triggering Hedgehog signaling.[3] |
|
| ln Vivo |
|
|
| Enzyme Assay | Smo binding assays are carried out using BODIPY-cyclopamine and Smo-overexpressing cells as previously described4,5. Expression constructs for Smo-Myc3, Smo⁄CRD (deletion of amino acids 68 to 182), and SmoCT (deletion of amino acids 556 to 793) are based on the CMV promoter and contain the SV40 origin. According to the manufacturer, HEK 293T cells are cultured on poly-D-lysine-treated glass coverslips in 12-well plates until 70% confluency. After that, they are transfected using FuGene 6 with the appropriate expression construct (0.5 g/well). | |
| Cell Assay | In T175 flasks, C3H10T1/2 cells are cultivated; at the thirteenth passage, the cells are separated using trypsin/EDTA and then diluted in the cultures. A Multi-dropTM liquid delivery system is then used to plate the resultant cell suspension into black clear bottom 384-well plates with 2500 cells/well in 100 µL growth medium. Cells adhered to the bottom of the wells following an overnight incubation. A Mini TrakTM multiposition dispenser system is used to transfer a 500 nL stock solution of each Purmorphamine in DMSO into the corresponding well, resulting in a final concentration of 5μM Purmorphamine. After that, the cells are incubated in an air atmosphere at 37 °C with 5% CO2. After four days, each well receives 10 μL of passive lysis buffer after the medium is removed. Ten microliters of alkaline phosphatase substrate solution are added to each well after five minutes. The plates are read using an Acquest high-throughput plate reader in accordance with the manufacturer's instructions after being incubated for 15 minutes at room temperature. | |
| Animal Protocol |
Male C57BL/6J mouse pups 10 mg/kg i.p. |
|
| References |
[1]. Nat Chem Biol . 2006 Jan;2(1):29-30. [2]. J Am Chem Soc . 2002 Dec 11;124(49):14520-1. [3]. Chem Biol . 2004 Sep;11(9):1229-38. [4]. Biomed Pharmacother . 2013 Feb;67(1):31-8. [5]. Front Pharmacol . 2020 Mar 4:11:204. |
|
| Additional Infomation | Purmorphamine is a member of the class of purines that is purine substituted at C-2 by a 1-naphthyloxy group, at C-4 by a 4-morpholinophenylamino group, and at N-9 by a cyclohexyl group. It has a role as an osteogenesis regulator and a SMO receptor agonist. It is a member of purines, a member of morpholines, an aromatic ether and a secondary amino compound. It derives from a hydride of a 9H-purine. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.80 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.80 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (4.80 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 2% DMSO+30% PEG 300+5% Tween 80+ddH2O: 1 mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9208 mL | 9.6039 mL | 19.2079 mL | |
| 5 mM | 0.3842 mL | 1.9208 mL | 3.8416 mL | |
| 10 mM | 0.1921 mL | 0.9604 mL | 1.9208 mL |