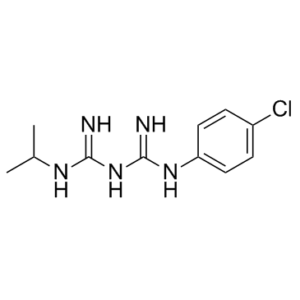
Proguanil, also known as chlorguanide and chloroguanide, is a orally available medication used to treat and prevent malaria. It is an antimalarial prodrug that is metabolized to the active metabolite cycloguanil, a dihydrofolate reductase (DHFR) inhibitor. It is often used together with chloroquine or atovaquone. When used with chloroquine the combination will treat mild chloroquine resistant malaria. When used alone, proguanil functions as a prodrug. Its active metabolite, cycloguanil, is an inhibitor of dihydrofolate reductase (DHFR). Although both mammals and parasites produce DHFR, cycloguanil's inhibitory activity is specific for parasitic DHFR. This enzyme is a critical component of the folic acid cycle. Inhibition of DHFR prevents the parasite from recycling dihydrofolate back to tetrahydrofolate (THF). THF is required for DNA synthesis, amino acid synthesis, and methylation; thus, DHFR inhibition shuts down these processes.
Physicochemical Properties
| Molecular Formula | C11H16CLN5 | |
| Molecular Weight | 253.73 | |
| Exact Mass | 253.109 | |
| Elemental Analysis | C, 52.07; H, 6.36; Cl, 13.97; N, 27.60 | |
| CAS # | 500-92-5 | |
| Related CAS # | Proguanil-d6;Proguanil hydrochloride;637-32-1;Proguanil-d4;1189805-15-9 | |
| PubChem CID | 6178111 | |
| Appearance | Solid powder | |
| Density | 1.29g/cm3 | |
| Boiling Point | 402.7ºC at 760mmHg | |
| Melting Point | 129° | |
| Flash Point | 197.4ºC | |
| Index of Refraction | 1.6110 (estimate) | |
| LogP | 3.263 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 1 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 17 | |
| Complexity | 292 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | ClC1C([H])=C([H])C(=C([H])C=1[H])N([H])/C(/N([H])[H])=N/C(/N([H])[H])=N/C([H])(C([H])([H])[H])C([H])([H])[H] |
|
| InChi Key | SSOLNOMRVKKSON-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C11H16ClN5/c1-7(2)15-10(13)17-11(14)16-9-5-3-8(12)4-6-9/h3-7H,1-2H3,(H5,13,14,15,16,17) | |
| Chemical Name | Biguanide, 1-(p-chlorophenyl)-5-isopropyl- | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Plasmodium |
| ln Vitro |
Proguanil's efficacy against malaria in vitro is primarily dependent on Cycloguanil, its active metabolite, which has a much stronger antimalarial activity (IC50=2.4-19 μM). An inhibitor of dihydrofolate reductase (DHFR) is Cycloguanil. In vitro, the combination of proguanil and atovaquone works well together. Both medications are effective against malaria parasites in their pre-erythrocytic (hepatic) stages as well as gametocytes[1]. To increase the effects of atovaquone, proguanil functions as a biguanide instead of its metabolite, cycloguanil, which is an inhibitor of the parasite dihydrofolate reductase [DHFR]. Since proguanil does not change the effects of other mitochondrial electron transport inhibitors, such as myxothiazole and antimycin, its enhancement of atovaquone is specific[2]. Proguanil, 4-chlorophenyl-1-biguanide (CPB), and Cycloguanil (CG), the active metabolite, all reversibly inhibit 5-HT3 receptor responses with IC50 values of 1.81, 1.48, and 4.36 μM, respectively[3]. |
| ln Vivo |
Proguanil (p.o.; 2.9 mg/kg body weight; daily for 5 days and 6 weeks, respectively) causes mild degenerative changes in wistar strain albino rats for 5 days and severe degenerative changes for 6 weeks.Rats receiving proguanil treatment show a significant decrease in serum testosterone levels[4]. When Malarone (atovaquone and proguanil) is given to experimentally infected dogs with B. gibsoni in two chronic stages and three acute stages, the parasitemia levels drop and clinical improvements are seen[5]. |
| Cell Assay | Sertoli cells from sixteen to eighteen-day-old rats are cultured and exposed to proguanil at concentrations of 0.3 μM to 10 μM for five days. The viability and integrity of the Sertoli cells' nuclei are then assessed. Additionally, transferrin and Glial cell line-derived neurotrophic factor's genetic expressions are evaluated[4]. |
| Animal Protocol | Rats: Proguanil (2.9 mg/kg body weight) is given daily to groups of ten to twelve-week-old rats for five days and six weeks, respectively. Following that, weights of the body and reproductive organs are recorded, sperm parameters are examined, and testicular and epididymal histology is performed. Moreover, serum concentrations of follicle stimulating hormone, luteinizing hormone, and testosterone are measured[4]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Rapidly and well absorbed in humans following oral doses ranging from 50 to 500 mg. Metabolism / Metabolites Variably metabolized in the liver by cytochrome P450 isoenzymes to the active triazine metabolite, cycloguanil. This variable metabolism of proguanil may have profound clinical importance in poor metabolizers such as the Asian and African populations at risk for malaria infection. Prophylaxis with proguanil may not be effective in these persons because they may not achieve adequate therapeutic levels of the active compound, cycloguanil, even after multiple doses. Proguanil has known human metabolites that include Cycloguanil and 4-Chlorophenylbiguanide. Biological Half-Life Approximately 20 hours |
| Toxicity/Toxicokinetics |
Hepatotoxicity The combination of atovaquone and proguanil has been associated with transient and minor serum aminotransferase elevations in a small proportion of patients. More importantly, there have been rare reports of idiosyncratic acute liver injury due in patients on atovaquone/ proguanil but the number of cases has been too few to define a typical clinical course. In one reported case, the onset of injury was after 3 weeks and presentation was with fatigue and jaundice and a cholestatic pattern of serum enzyme elevations. The injury resolved within 2 months of stopping the medication (Case 1). In another case report of chloroquine and proguanil, liver injury arose within days of starting the combination and the pattern of serum enzyme elevations was mixed. In both cases, allergic features were minimal and autoantibodies were not present. In both cases, combination therapy was used and either agent may have been the cause of the injury. Atovaquone and proguanil have also been linked to rare instances of Stevens Johnson syndrome which is often accompanied by mild liver injury or liver enzyme elevations. Likelihood score: E* (unproven but sometimes suspected cause of clinically apparent liver injury). Protein Binding Approximately 75% |
| References |
[1]. Atovaquone and proguanil hydrochloride: a review of nonclinical studies. J Travel Med. 1999 May;6 Suppl 1:S8-12. [2]. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob Agents Chemother. 1999 Jun;43(6):1334-9. [3]. The antimalarial drug proguanil is an antagonist at 5-HT3 receptors. J Pharmacol Exp Ther. 2014 Dec;351(3):674-84. [4]. Prolonged administration of proguanil induces reproductive toxicity in male rats. J Toxicol Sci. 2011 Oct;36(5):587-99. [5]. The in vitro interactions and in vivo efficacy of atovaquone and proguanil against Babesia gibsoni infection in dogs. Vet Parasitol. 2013 Nov 8;197(3-4):527-33. |
| Additional Infomation |
Proguanil is a biguanide compound which has isopropyl and p-chlorophenyl substituents on the terminal N atoms. A prophylactic antimalarial drug, it works by inhibiting the enzyme dihydrofolate reductase, which is involved in the reproduction of the malaria parasites Plasmodium falciparum and P. vivax within the red blood cells. It has a role as an antimalarial, an antiprotozoal drug and an EC 1.5.1.3 (dihydrofolate reductase) inhibitor. It is a member of biguanides and a member of monochlorobenzenes. Proguanil is a prophylactic antimalarial drug, which works by stopping the malaria parasite, Plasmodium falciparum and Plasmodium vivax, from reproducing once it is in the red blood cells. It does this by inhibiting the enzyme, dihydrofolate reductase, which is involved in the reproduction of the parasite. Proguanil is an Antimalarial. The mechanism of action of proguanil is as a Dihydrofolate Reductase Inhibitor. Proguanil is a biguanide derivative which is active against several protozoal species and is used in combination with atovaquone and chloroquine for the prevention and therapy of malaria. Proguanil has not been evaluated extensively as a single agent, but the combinations of proguanil with atovaquone or chloroquine have been used to treat malaria and have been linked to serum enzyme elevations during therapy and rare instances of clinically apparent acute liver injury. A biguanide compound which metabolizes in the body to form cycloguanil, an anti-malaria agent. Drug Indication For the causal prevention and suppression of malaria caused by susceptible strains of P. falciparum and other species of Plasmodium found in some geographical areas of the world. Mechanism of Action Proguanil inhibits the dihydrofolate reductase of plasmodia and thereby blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication. This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver. Pharmacodynamics Proguanil is a biguanide derivative that is converted to an active metabolite called cycloguanil. It exerts its antimalarial action by inhibiting parasitic dihydrofolate reductase enzyme. It has causal prophylactic and suppressive activity against P. falciparum and cures the acute infection. It is also effective in suppressing the clinical attacks of vivax malaria. However it is slower compared to 4-aminoquinolines. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 51~130 mg/mL ( 201.0~512.36 mM ) Ethanol : ~51 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.17 mg/mL (8.55 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 21.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.17 mg/mL (8.55 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 21.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.17 mg/mL (8.55 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 21.7 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.17 mg/mL (8.55 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9412 mL | 19.7060 mL | 39.4120 mL | |
| 5 mM | 0.7882 mL | 3.9412 mL | 7.8824 mL | |
| 10 mM | 0.3941 mL | 1.9706 mL | 3.9412 mL |