Physicochemical Properties
| Molecular Formula | C30H26O12 |
| Molecular Weight | 578.5202 |
| Exact Mass | 578.142 |
| CAS # | 29106-49-8 |
| Related CAS # | Cyanidin Chloride;528-58-5;Procyanidin B1;20315-25-7;Procyanidin C1;37064-30-5;Procyanidin A2;41743-41-3;Procyanidin A1;103883-03-0;Procyanidin B3;23567-23-9 |
| PubChem CID | 122738 |
| Appearance | Light yellow to light brown solid powder |
| Density | 1.7±0.1 g/cm3 |
| Boiling Point | 955.3±65.0 °C at 760 mmHg |
| Melting Point | 197-198ºC |
| Flash Point | 531.6±34.3 °C |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.803 |
| LogP | 0.3 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 42 |
| Complexity | 925 |
| Defined Atom Stereocenter Count | 5 |
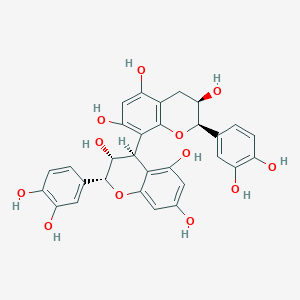
| SMILES | C1[C@H]([C@H](OC2=C1C(=CC(=C2[C@@H]3[C@H]([C@H](OC4=CC(=CC(=C34)O)O)C5=CC(=C(C=C5)O)O)O)O)O)C6=CC(=C(C=C6)O)O)O |
| InChi Key | XFZJEEAOWLFHDH-NFJBMHMQSA-N |
| InChi Code | InChI=1S/C30H26O12/c31-13-7-20(37)24-23(8-13)41-29(12-2-4-16(33)19(36)6-12)27(40)26(24)25-21(38)10-17(34)14-9-22(39)28(42-30(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,22,26-29,31-40H,9H2/t22-,26-,27-,28-,29-/m1/s1 |
| Chemical Name | (2R,3R)-2-(3,4-dihydroxyphenyl)-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Proanthocyanidin B2 shows anti-proliferative effect on MCF-7 cells with an IC50 of 19.21 μM. However, proanthocyanidin B2 has little influence on DNA ladder formation [1]. Proanthocyanidin B2 (0.1, 1, 2 μM) inhibits activation of the pyrin domain-containing 3 (NLRP3) inflammasome in returning human vascular venous ECs (HUVECs) by decreasing AP-1 activity, which can Overexpression of c-Jun was removed in the following manner. Proanthocyanidin B2 (2 μM for 12 hours) similarly decreases ROS in HUVECs [2]. |
| ln Vivo | Brain edema-induced infarct volume and cerebral edema are prevented by proanthocyanidin B2 (40, 20, and 10 mg/kg, Bay). Following cerebral ischemia, proanthocyanidin B2 (40 mg/kg, po) also enhances functional results and modifies blood-brain barrier (BBB) permeability. Furthermore, proanthocyanidin B2 has the ability to reduce intracellular oxidation, intermediate elimination, and connection breakage brought on by cerebral ischemia. In a normal brain in vivo, proanthocyanidin B2 (40 mg/kg, epidermal) enhances Nrf2 activation and the production of HO-1, GSTα, and NQO1 proteins [3]. |
| References |
[1]. Procyanidin b2 cytotoxicity to mcf-7 human breast adenocarcinoma cells. Indian J Pharm Sci. 2012 Jul;74(4):351-5. [2]. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem Pharmacol. 2014 Dec 15;92(4):599-606. [3]. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol Nutr Food Res. 2015 Oct;59(10):1930-41. |
| Additional Infomation |
Procyanidin B2 is a proanthocyanidin consisting of two molecules of (-)-epicatechin joined by a bond between positions 4 and 8' in a beta-configuration. Procyanidin B2 can be found in Cinchona pubescens (Chinchona, in the rind, bark and cortex), in Cinnamomum verum (Ceylon cinnamon, in the rind, bark and cortex), in Crataegus monogyna (Common hawthorn, in the flower and blossom), in Uncaria guianensis (Cat's claw, in the root), in Vitis vinifera (Common grape vine, in the leaf), in Litchi chinensis (litchi, in the pericarp), in the apple, in Ecdysanthera utilis and in red wine. It has a role as a metabolite and an antioxidant. It is a hydroxyflavan, a proanthocyanidin, a biflavonoid and a polyphenol. It is functionally related to a (-)-epicatechin. Procyanidin B2 has been reported in Camellia sinensis, Camellia reticulata, and other organisms with data available. See also: Primula veris flower (part of). |
Solubility Data
| Solubility (In Vitro) |
H2O : ~66.67 mg/mL (~115.24 mM) DMSO : ≥ 50 mg/mL (~86.43 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.32 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.32 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (4.32 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 50 mg/mL (86.43 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7285 mL | 8.6427 mL | 17.2855 mL | |
| 5 mM | 0.3457 mL | 1.7285 mL | 3.4571 mL | |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7285 mL |