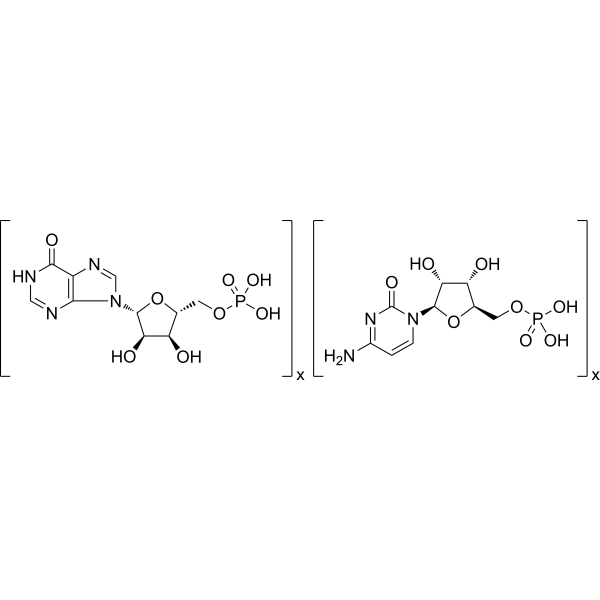
Physicochemical Properties
| Molecular Formula | (C10H13N4O8P)X.(C9H14N3O8P)X |
| Molecular Weight | 671.4025 |
| Exact Mass | 671.098 |
| CAS # | 24939-03-5 |
| Related CAS # | Poly (I:C):Kanamycin (1:1);Polyinosinic-polycytidylic acid potassium;31852-29-6;Polyinosinic-polycytidylic acid sodium;42424-50-0 |
| PubChem CID | 135478809 |
| Appearance | White to off-white solid powder |
| Boiling Point | 851.4ºC at 760 mmHg |
| Vapour Pressure | 7.7E-31mmHg at 25°C |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 44 |
| Complexity | 1090 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | ACEVNMQDUCOKHT-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C10H13N4O8P.C9H14N3O8P/c15-6-4(1-21-23(18,19)20)22-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17;10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h2-4,6-7,10,15-16H,1H2,(H,11,12,17)(H2,18,19,20);1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18) |
| Chemical Name | [5-(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate;[3,4-dihydroxy-5-(6-oxo-1H-purin-9-yl)oxolan-2-yl]methyl dihydrogen phosphate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | The paracellular permeability of immortalized airway epithelial cells is increased at a dose and time by polyinosinic-polycytidylic acid (0.5–5 μg/mL, 3–24 h) [4]. 16HBE14o- cells experience disruption of their epithelial apical junction complexes and tight junctions when exposed to polyinosinic-polycytidylic acid (5 μg/mL, 6 h) [4]. However, the acid does not cause any cytotoxicity to 16HBE14o- cells [4]. |
| ln Vivo | Polyinosinic acid (2.5-10 mg/mL, stereotactic injection, single dosage) causes prolonged reactive reactions in the substantia nigra and dorsal striatum [2]. Polyinosinic-polycytidylic Acid (10 μg/mouse, i.p.) Polyinosinic-polycytidylic Acid (1.25 mg/kg, i.p., single dosage) boosts brain tumor lung growth via TLR3 and TLR4/MyD88 signaling in the MCAO model [3 ]. |
| Cell Assay |
Cell Cytotoxicity Assay[4] Cell Types: 16HBE14o- Cell Tested Concentrations: 5 μg/mL Incubation Duration: 24 hrs (hours) Experimental Results: No significant accumulation of LDH in the cell culture medium |
| Animal Protocol |
Animal/Disease Models: lung tumor mice [3] Doses: 10 μg/mouse Route of Administration: intraperitoneal (ip) injection Experimental Results: The growth of lung metastases in tumor-bearing mice was Dramatically diminished. . The number of lung lesions was diminished to approximately 40%. The number of BAL fluid cells increased Dramatically. Increased levels of INF-γ and IL-17A and diminished levels of IL-13. Increased TLR3 expression. Animal/Disease Models: Middle cerebral artery occlusion (MCAO) model mouse [5] Doses: 1.25 mg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results: Reduce focal brain I/R injury. Increase the expression of Bcl2, Hsp27 and Hsp70, reduce Bax expression, and reduce cell degeneration and apoptosis. Downregulating TLR4 signaling through TLR3 prevents cerebral ischemia and provides protection against cerebral I/R injury. |
| References |
[1]. Cheng Y, Xu F. Anticancer function of polyinosinic-polycytidylic acid . Cancer biology & therapy, 2010, 10(12): 1219-1223. [2]. The Toll-like receptor-3 agonist polyinosinic: polycytidylic acid triggers nigrostriatal dopaminergic degeneration . Journal of Neuroscience, 2010, 30(48): 16091-16101. [3]. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer . The Journal of Immunology, 2012, 188(11): 5357-5364. [4]. Polyinosinic: polycytidylic acid induces protein kinase D–dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells . Journal of Allergy and Clinical Immunology, 2011, 128(6): 1216-1224. e11. [5]. Polyinosinic-polycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3 . The Journal of Immunology, 2014, 192(10): 4783-4794. [6]. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732-738. [7]. Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002;293(5):1364-1369. |
Solubility Data
| Solubility (In Vitro) | H2O : ~50 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 100 mg/mL (Infinity mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4894 mL | 7.4471 mL | 14.8943 mL | |
| 5 mM | 0.2979 mL | 1.4894 mL | 2.9789 mL | |
| 10 mM | 0.1489 mL | 0.7447 mL | 1.4894 mL |