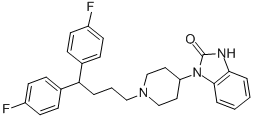
Pimozide (R-6238; NSC-170984; Orap) is an antipsychotic drug of the diphenylbutylpiperidine class discovered y Janssen Pharmaceuticals in 1963. Pimozide is an antagonist of dopamine receptors, with Kis values of 1.4 nM, 2.5 nM, and 588 nM for dopamine D2, D3, and D1 receptors, respectively. It also exhibits affinity at the α1-adrenoceptor, with a Ki of 39 nM. When compared to chlorpromazine, pimozide is much more potent (ratio 50–70:1). It is even more effective than haloperidol when compared weight for weight. Additionally, it has specific neurologic indications for resistant tics and Tourette syndrome. Akathisia, tardive dyskinesia, and, in rarer cases, neuroleptic malignant syndrome and prolongation of the QT interval are among the side effects.
Physicochemical Properties
| Molecular Formula | C28H29F2N3O |
| Molecular Weight | 461.55 |
| Exact Mass | 461.227 |
| Elemental Analysis | C, 72.86; H, 6.33; F, 8.23; N, 9.10; O, 3.47 |
| CAS # | 2062-78-4 |
| Related CAS # | Pimozide-d4-1; Pimozide-d4; 1803193-57-8; Pimozide-d5 N-Oxide; 1794795-40-6 |
| PubChem CID | 16362 |
| Appearance | White solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 649.0±65.0 °C at 760 mmHg |
| Melting Point |
214-218 °C 214 - 218 °C |
| Flash Point | 346.3±34.3 °C |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.623 |
| LogP | 6.06 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 34 |
| Complexity | 632 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C1NC2=CC=CC=C2N1C3CCN(CCCC(C4=CC=C(F)C=C4)C5=CC=C(F)C=C5)CC3 |
| InChi Key | YVUQSNJEYSNKRX-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) |
| Chemical Name | 3-[1-[4,4-bis(4-fluorophenyl)butyl]piperidin-4-yl]-1H-benzimidazol-2-one |
| Synonyms | R6238; NSC 170984; NSC170984; R-6238; NSC-170984; R 6238 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Dopamine D2 receptor ( Ki = 1.4 nM ); Dopamine D3 receptor ( Ki = 2.5 nM ); Dopamine D1 receptor ( Ki = 588 nM ); α1-adrenoceptor ( Ki = 39 nM ); STAT3; STAT5 | |
| ln Vitro |
|
|
| Cell Assay |
|
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Greater than 50% absorption after oral administration. Serum peak appears 6-8 hours post ingestion. Metabolism / Metabolites Notable first-pass metabolism in the liver, primarily by N-dealkylation via the cytochrome P450 isoenzymes CYP3A and CYP1A2 (and possibly CYP2D6). The activity of the two major metabolites has not been determined. Pimozide has known human metabolites that include 1,3- dihydro-1-(4-piperidinyl)-2H-benzimidazol-2-one (DHPBI). Notable first-pass metabolism in the liver, primarily by N-dealkylation via the cytochrome P450 isoenzymes CYP3A and CYP1A2 (and possibly CYP2D6). The activity of the two major metabolites has not been determined. Half Life: 29 ± 10 hours (single-dose study of healthy volunteers). Biological Half-Life 29 ± 10 hours (single-dose study of healthy volunteers). |
|
| Toxicity/Toxicokinetics |
Toxicity Summary The ability of pimozide to suppress motor and phonic tics in Tourette's Disorder is thought to be primarily a function of its dopaminergic blocking activity. Pimozide binds and inhibits the dopamine D2 receptor in the CNS. It also appears to block voltage-operated calcium channels and acts as an antagonist at opiate receptors (OP2). Hepatotoxicity Liver test abnormalities have not been reported to occur in of patients on pimozide, but the degree and duration of monitoring done in initial studies were not clear. Instances of clinically apparent acute liver injury have not been reported due to pimozide, and thus must be rare if they occur at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because there is no published experience with pimozide during breastfeeding, other drugs are preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Pimozide can cause hyperprolactinemia. The hyperprolactinemia is caused by the drug's dopamine-blocking action in the tuberoinfundibular pathway. Toxicity Data LD50: 1100 mg/kg (Oral, Rat) (A308) LD50: 228 mg/kg (Oral, Mouse) (A308) |
|
| References |
[1]. Adrenoceptors and dopamine receptors are not involved in the discriminative stimulus effect of the 5-HT1A receptor agonist flesinoxan. Eur J Pharmacol. 1994 Apr 21;256(2):141-7. [2]. The STAT3 inhibitor pimozide impedes cell proliferation and induces ROS generation in human osteosarcoma by suppressing catalase expression. Am J Transl Res. 2017 Aug 15;9(8):3853-3866. eCollection 2017. [3]. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011 Mar 24; 117(12): 3421-3429 |
|
| Additional Infomation |
Pimozide can cause developmental toxicity and female reproductive toxicity according to state or federal government labeling requirements. Pimozide is a member of the class of benzimidazoles that is 1,3-dihydro-2H-benzimidazol-2-one in which one of the nitrogens is substituted by a piperidin-4-yl group, which in turn is substituted on the nitrogen by a 4,4-bis(p-fluorophenyl)butyl group. It has a role as a H1-receptor antagonist, a serotonergic antagonist, a first generation antipsychotic, an antidyskinesia agent and a dopaminergic antagonist. It is a member of benzimidazoles, an organofluorine compound and a heteroarylpiperidine. A diphenylbutylpiperidine that is effective as an antipsychotic agent and as an alternative to haloperidol for the suppression of vocal and motor tics in patients with Tourette syndrome. Although the precise mechanism of action is unknown, blockade of postsynaptic dopamine receptors has been postulated. (From AMA Drug Evaluations Annual, 1994, p403) Pimozide is a Typical Antipsychotic. Pimozide is a conventional antipsychotic used largely in the therapy of Tourette syndrome. Pimozide therapy has not been associated with serum aminotransferase elevations nor with cases of clinically apparent acute liver injury. Pimozide is a diphenylbutylpiperidine derivative and a dopamine antagonist with antipsychotic property. Pimozide selectively inhibits type 2 dopaminergic receptors in the central nervous system (CNS), thereby decreasing dopamine neurotransmission and reducing the occurrence of motor and vocal tics and delusions of parasitosis. In addition, this agent antagonizes alpha-adrenergic and 5-HT2 receptors. A diphenylbutylpiperidine that is effective as an antipsychotic agent and as an alternative to haloperidol for the suppression of vocal and motor tics in patients with Tourette syndrome. Although the precise mechanism of action is unknown, blockade of postsynaptic dopamine receptors has been postulated. (From AMA Drug Evaluations Annual, 1994, p403) A diphenylbutylpiperidine that is effective as an antipsychotic agent and as an alternative to HALOPERIDOL for the suppression of vocal and motor tics in patients with Tourette syndrome. Although the precise mechanism of action is unknown, blockade of postsynaptic dopamine receptors has been postulated. (From AMA Drug Evaluations Annual, 1994, p403) Drug Indication Used for the suppression of motor and phonic tics in patients with Tourette's Disorder who have failed to respond satisfactorily to standard treatment. Mechanism of Action The ability of pimozide to suppress motor and phonic tics in Tourette's Disorder is thought to be primarily a function of its dopaminergic blocking activity. Pimozide binds and inhibits the dopamine D2 receptor in the CNS. Pharmacodynamics Pimozide is an orally active antipsychotic drug product which shares with other antipsychotics the ability to blockade dopaminergic receptors on neurons in the central nervous system. However, receptor blockade is often accompanied by a series of secondary alterations in central dopamine metabolism and function which may contribute to both pimozide's therapeutic and untoward effects. In addition, pimozide, in common with other antipsychotic drugs, has various effects on other central nervous system receptor systems which are not fully characterized. Pimozide also has less potential for inducing sedation and hypotension as it has more specific dopamine receptor blocking activity than other neuroleptic agents (and is therefore a suitable alternative to haloperidol). |
Solubility Data
| Solubility (In Vitro) |
DMSO: 16.7~90 mg/mL (36.1~195 mM) H2O: < 0.1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.42 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 2: ≥ 1.67 mg/mL (3.62 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: 1.67 mg/mL (3.62 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1666 mL | 10.8331 mL | 21.6661 mL | |
| 5 mM | 0.4333 mL | 2.1666 mL | 4.3332 mL | |
| 10 mM | 0.2167 mL | 1.0833 mL | 2.1666 mL |