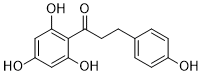
Phloretin (also known as NSC 407292 and RJC 02792), a naturally occuring dihydrochalcone flavonoid mainly found in fruit, leaves, and roots of apple tree, inhibits a variety of transporters such as the monocarboxylate transporters MCT1 and MCT2 (IC50 = 28 and 14 µM, respectively). Phloretin has anti-inflammatory, anti-tumor, and antioxidant properties in mammalian cells. Phloretin(NSC 407292; RJC 02792) is a dihydrochalcone, a type of natural phenols. Phloretin blocks SGLT1 and SGLT2's ability to actively transport glucose into cells.
Physicochemical Properties
| Molecular Formula | C15H14O5 |
| Molecular Weight | 274.2687 |
| Exact Mass | 274.084 |
| Elemental Analysis | C, 65.69; H, 5.15; O, 29.17 |
| CAS # | 60-82-2 |
| PubChem CID | 4788 |
| Appearance | Off-white to pink solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 534.4±29.0 °C at 760 mmHg |
| Melting Point | ~260 °C |
| Flash Point | 291.1±20.8 °C |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.685 |
| LogP | 3.5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 20 |
| Complexity | 312 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(C1C(=C([H])C(=C([H])C=1O[H])O[H])O[H])C([H])([H])C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])O[H] |
| InChi Key | VGEREEWJJVICBM-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C15H14O5/c16-10-4-1-9(2-5-10)3-6-12(18)15-13(19)7-11(17)8-14(15)20/h1-2,4-5,7-8,16-17,19-20H,3,6H2 |
| Chemical Name | 3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one |
| Synonyms | RJC 02792; NSC 407292; NSC-407292; NSC407292; RJC02792; RJC-02792; Phloretin |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | SGLT1; SGLT2; Microbial Metabolite; GLUT1; GLUT2 |
| ln Vitro | Phloretin is a dihydrochalcone that can be found in the bark of fruit trees such as cherries, apples, and pears (Pyrus communis). Phloretin, like its glycoside phlorizin, inhibits the active transport of glucose into cells by SGLT1 and SGLT2, albeit to a lesser extent. [1] In the small intestine, hydrolytic enzymes convert nearly all of the phlorizin taken orally into phloretin. An important effect of this is the inhibition of glucose absorption by the small intestine and the inhibition of renal glucose reabsorption. [2] [3] Additionally, a number of urea transporters are inhibited by phenyletin. When used in conjunction with diets high in protein, it causes diuresis and urea loss. [4] |
| ln Vivo | Phloretin (methanol; 50 or 100 mg/kg; once daily) reduces hydrogen peroxide and malondialdehyde (MDA) and hydrogen peroxide (H2O2) levels in paw tissue and reduces anticollagen efficacy in serum [animal model : Collagen-induced arthritis (CIA) mice [3] Dosage: 50 or 100 mg/kg Administration method: Oral Results: Compared with traditional drugs, in addition to reducing inflammation in the hind limbs, it also showed relief from clinical symptoms of RA. 3]. control group. |
| Cell Assay |
Cell Line: BEL-7402 cell Concentration: 40-160 μM Incubation Time: 24 hours Result: Induced cell apoptosis and activated caspase 3, 6 and 9. |
| Animal Protocol |
Collagen-Induced Arthritis (CIA) Mice 50 or 100 mg/kg Oral adminstration |
| ADME/Pharmacokinetics |
Metabolism / Metabolites Phloretin has known human metabolites that include Phloretin, 4p-hydroxy-glucuronide and Phloretin, 2p-hydroxy-glucuronide. |
| References |
[1]. Am J Physiol . 1962 Dec:203:975-9. [2]. Diabetes Obes Metab . 2009 Feb;11(2):79-88. [3]. J Nutr . 2001 Dec;131(12):3227-30. [4]. Proc Natl Acad Sci U S A . 2004 May 11;101(19):7469-74. |
| Additional Infomation |
Phloretin is a member of the class of dihydrochalcones that is dihydrochalcone substituted by hydroxy groups at positions 4, 2', 4' and 6'. It has a role as a plant metabolite and an antineoplastic agent. It is functionally related to a dihydrochalcone. Phloretin is a natural dihydrochalcone found in apples and many other fruits. Phloretin has been reported in Malus, Boesenbergia rotunda, and other organisms with data available. A natural dihydrochalcone found in apples and many other fruits. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 50~55 mg/mL (182.3~200.5 mM) Ethanol: ~55 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.12 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (9.12 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (9.12 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 5%DMSO + Corn oil: 0.45mg/ml (1.64mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6460 mL | 18.2302 mL | 36.4604 mL | |
| 5 mM | 0.7292 mL | 3.6460 mL | 7.2921 mL | |
| 10 mM | 0.3646 mL | 1.8230 mL | 3.6460 mL |