Physicochemical Properties
| Molecular Formula | C8H8O2 |
| Molecular Weight | 136.15 |
| Exact Mass | 136.052 |
| CAS # | 122-79-2 |
| Related CAS # | Phenyl acetate-d5;22705-26-6 |
| PubChem CID | 31229 |
| Appearance | Colorless to light yellow liquid |
| Density | 1.073 g/mL at 25 °C(lit.) |
| Boiling Point | 196 °C(lit.) |
| Melting Point | 195-196℃ |
| Flash Point | 170 °F |
| Vapour Pressure | 0.418mmHg at 25°C |
| Index of Refraction | n20/D 1.501(lit.) |
| LogP | 1.611 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 10 |
| Complexity | 114 |
| Defined Atom Stereocenter Count | 0 |
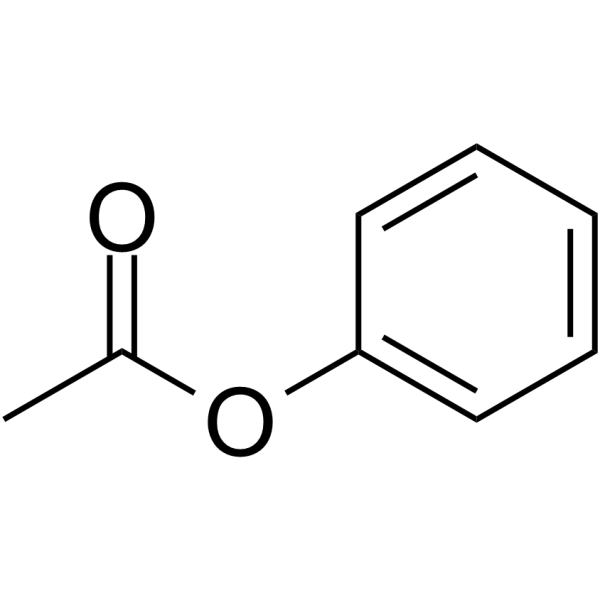
| SMILES | CC(=O)OC1=CC=CC=C1 |
| InChi Key | IPBVNPXQWQGGJP-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C8H8O2/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3 |
| Chemical Name | phenyl acetate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ADME/Pharmacokinetics |
Metabolism / Metabolites PHENYL ACETATE /WAS METABOLIZED TO/ PHENOL, IN MAN; IN PIG; IN RABBIT; AND IN PEA. The substrate specificity of carboxylesterases (CEs) in various respiratory tract tissues from F344/N-rats, New-Zealand-white-rabbits, and Syrian-hamsters was studied using ion chromatography to quantify hydrolysis rates. Thirteen esters were tested: pentyl-acetate, phenyl-acetate and beta-butyrolactone were used for interspecies comparison. Liver S9 had the most catalytic activity in rats whereas in rabbits and hamsters the values for trachea and nasal S9 were as active or more active than liver for all substrates tested except pentyl-acetate. Lung S9 showed reduced esterase activity relative to the other tissues. ... Straight chain alcohol esters were the most rapidly hydrolyzed and tertiary esters had the slowest hydrolysis rate. ... The substrate specificity for carboxylic acid esters was determined for phosphoric triester hydrolases and serine esterases. Serum was obtained from 452 individuals, including non diseased person and patients with hyperlipemia. Correlations between enzyme activities, reversible inhibition by EDTA and progressive inhibition by organophosphate compounds and carbamates were made. The hydrolysis of paraoxon (POX), phenylacetate (PA), and beta-naphthylacetate (BNA) was studied. Results indicated that two paraoxonases hydrolyze paraoxon, one sensitive and the other insensitive to EDTA. The EDTA sensitive paraoxonase also hydrolyzed beta-naphthylacetate. The EDTA insensitive hydrolysis of beta-naphthylacetate and phenylacetate was assigned to a serine esterase. The EDTA sensitive hydrolysis of phenylacetate was likely due to more than one enzyme, which may be an arylesterase and a carboxylesterase. Esterases in human liver microsomes hydrolyzed ... phenylacetate (Vmax 57 +/- 8 mumol/min/g tissue), whereas esterases found in the human liver cytosol hydrolyzed ... phenylacetate (Vmax 37 +/- 2.9 mumol/min/g tissue). ... Human plasma esterase hydrolyzed ... phenylacetate (Vmax 250 +/- 17 mumol/min/mL). ... Phenylacetate hydrolysis involved arylesterase in plasma, both arylesterase and carboxylesterase in liver microsomes and carboxylesterase in liver cytosol. ... For more Metabolism/Metabolites (Complete) data for PHENYL ACETATE (6 total), please visit the HSDB record page. |
| Additional Infomation |
Phenol acetate appears as a clear colorless liquid with a sweetish solvent odor. Difficult to ignite. Used as a laboratory reagent and in the production of some organic chemicals. Phenyl acetate is an acetate ester obtained by the formal condensation of phenol with acetic acid. It is a member of phenyl acetates and a member of benzenes. It is functionally related to a phenol. Phenyl acetate has been reported in Arabidopsis thaliana and Euglena gracilis with data available. Phenyl Acetate is an aromatic fatty acid metabolite of phenylalanine with potential antineoplastic activity. Naturally occurring in mammals, phenylacetate induces differentiation, growth inhibition, and apoptosis in tumor cells. Implicated mechanisms of action include decreased protein prenylation, activation of the peroxisome proliferation-activated receptors, inhibition of DNA methylation, and depletion of glutamine. (NCI04) Phenyl acetate is a metabolite found in or produced by Saccharomyces cerevisiae. |
Solubility Data
| Solubility (In Vitro) | DMSO: 250 mg/mL (1836.21 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (15.28 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (15.28 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (15.28 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.3448 mL | 36.7242 mL | 73.4484 mL | |
| 5 mM | 1.4690 mL | 7.3448 mL | 14.6897 mL | |
| 10 mM | 0.7345 mL | 3.6724 mL | 7.3448 mL |