AZD2624 (also known as AZD-4901, MLE-4901, AZ-12472520, and AZD-2624) is a novel, potent, selective and orally bioactive neurokinin-3 receptor (NK3R) antagonist. It was identified pharmacologically as an NK3 receptor antagonist with the goal of treating schizophrenia. In vitro studies were conducted to assess the potential for metabolic drug-drug interactions with AZD2624. It appeared that CYP3A4, CYP3A5, and CYP2C9 were the main enzymes involved in the formation of the hydroxylated metabolite (M2), while CYP3A4, CYP3A5, and CYP2C9 appeared to be the main enzymes mediating the formation of the pharmacologically active ketone metabolite (M1). For the formation of M1 and M2 in human liver microsomes, the apparent K(m) values were 1.5 and 6.3 µM, respectively. In the midazolam and testosterone assays, AZD2624 demonstrated an inhibitory effect on microsomal CYP3A4/5 activities, with apparent IC(50) values of 7.1 and 19.8 µM, respectively. AZD2624 did not appear to inactivate CYP3A4/5 activity (midazolam 1'-hydroxylation) in a time-dependent manner. CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6 showed little to no inhibition when exposed to AZD2624. CYP1A2 and CYP2B6 were not induced by AZD2624. Even though AZD2624 increased CYP3A4 activity in hepatocytes, at relevant exposure concentrations, there was little chance that AZD2624 would result in inductive drug interactions involving this enzyme. The study's findings showed that in addition to its targeted low efficacious concentration, AZD2624 has a comparatively low potential for metabolic drug-drug interactions with concurrently administered medications. On the other hand, AZD2624's metabolism may be impeded if strong CYP3A4/5 inhibitors are also taken concurrently.
Physicochemical Properties
| Molecular Formula | C26H25N3O3S |
| Molecular Weight | 459.56000494957 |
| Exact Mass | 459.162 |
| Elemental Analysis | C, 67.95; H, 5.48; N, 9.14; O, 10.44; S, 6.98 |
| CAS # | 941690-55-7 |
| PubChem CID | 23649245 |
| Appearance | White to off-white solid powder |
| LogP | 6.883 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 33 |
| Complexity | 740 |
| Defined Atom Stereocenter Count | 1 |
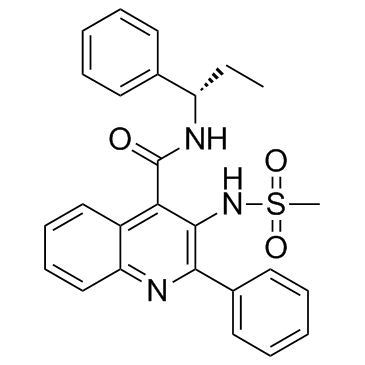
| SMILES | C(C1C2C=CC=CC=2N=C(C2C=CC=CC=2)C=1NS(=O)(=O)C)(=O)N[C@H](C1C=CC=CC=1)CC |
| InChi Key | QYTBBBAHNIWFOD-NRFANRHFSA-N |
| InChi Code | InChI=1S/C26H25N3O3S/c1-3-21(18-12-6-4-7-13-18)28-26(30)23-20-16-10-11-17-22(20)27-24(19-14-8-5-9-15-19)25(23)29-33(2,31)32/h4-17,21,29H,3H2,1-2H3,(H,28,30)/t21-/m0/s1 |
| Chemical Name | 3-(methanesulfonamido)-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-carboxamide |
| Synonyms | MLE-4901; MLE4901; MLE 4901; AZD-4901; AZD 4901; AZD4901; AZD-2624; AZD2624; AZD 2624; AZ-12472520; AZ 12472520; AZ12472520 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | NK3R |
| ln Vitro | Pavinetant (AZD2624) is a strong and focused antagonist of the NK3 receptor that was created to treat schizophrenia. The microsomal CYP3A4/5 activities are inhibited by the pavinetant, as demonstrated by apparent IC50 values of 19.8 μM for testosterone and 7.1 μM for midazolam assays, respectively. There is no evidence of a time-dependent Pavinetant-induced inactivation of CYP3A4/5 activity. Pavinetant inhibits CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6 weakly to nonexistently[1]. |
| Enzyme Assay | Pavinetant (AZD2624) is tested for its ability to inhibit CYP3A activities in a time-dependent manner by pre-incubating 10 μM of Pavinetant in 0.2 mL of 0.1 M pH 7.4 phosphate buffer, which contains 1 mM NADPH and 2 mg/mL HLM, at 37°C for 0, 3, 10, 20, and 30 minutes. Furthermore, as a positive control, verapamil is tested at 10 μM and incubated separately. For every time point, an aliquot of 20 μL is taken out of the pre-incubation tube and added to 180 μL of a secondary 5-min incubation that contains 1 mM NADPH and 15 μM midazolam. CYP3A enzymes are detected by LC-MS analysis of the 1′-hydroxymidazolam formation, which serves as a marker activity. The post-pre-incubation CYP3A enzyme activities with and without the Pavinetant pre-incubation are compared to the activities after the vehicle solvent (1% methanol) incubation[1]. |
| References |
[1]. In vitro assessment of metabolic drug–drug interaction potential of AZD2624, neurokinin-3 receptor antagonist, through cytochrome P(450) enzyme identification, inhibition, and induction studies. Xenobiotica. 2010 Nov;40(11):721-9. |
| Additional Infomation |
Pavinetant is a member of the class of quinolines that is the amide obtained by formal condensation of the carboxy group of 3-[(methanesulfonyl)amino]-2-phenylquinoline-4-carboxylic acid with the amino group of (1S)-1-phenylpropan-1-amine. A neurokinin-3 receptor antagonist that has been trialled as a potential drug for treatment of schizophrenia and menopausal symptoms. It has a role as a neurokinin-3 receptor antagonist and an antipsychotic agent. It is a member of quinolines, a secondary carboxamide, a sulfonamide and an aromatic amide. Pavinetant has been used in trials studying the basic science and treatment of Safety and Schizophrenia. |
Solubility Data
| Solubility (In Vitro) | DMSO: ≥ 50 mg/mL (~108.8 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (6.53 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3 mg/mL (6.53 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1760 mL | 10.8800 mL | 21.7599 mL | |
| 5 mM | 0.4352 mL | 2.1760 mL | 4.3520 mL | |
| 10 mM | 0.2176 mL | 1.0880 mL | 2.1760 mL |