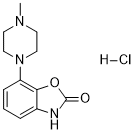
Pardoprunox (formerly known as SLV-308, DU-126891 or SME-308) is novel & potent dopamine D2/5-HT1A receptor agonist that has the potential for the treatment of Parkinson's disease. Pardoprunox functions by binding to 5-HT(1) (A) and dopamine D(2), D(3), and D(4) receptors.It is a full agonist at serotonin 5-HT(1) (A) receptors and a partial agonist at dopamine D(2) and D(3) receptors. SLV308 functioned as a strong but partial D(2) receptor agonist at cloned human dopamine D(2,L) receptors (pEC(50) = 8.0 and pA(2) = 8.4) with a 50% efficacy on forskolin stimulated cAMP accumulation. SLV308 functioned as a partial agonist at human recombinant dopamine D(3) receptors, inducing [(35)S]GTPgammaS binding (intrinsic activity of 67%; pEC(50) = 9.2) and inhibiting the dopamine-induced [(35)S]GTPgammaS binding (pA(2) = 9.0). SLV308 had low potency (pEC(50) = 6.3) but nonetheless functioned as a complete 5-HT(1) (A) receptor agonist on forskolin-induced cAMP accumulation at cloned human 5-HT(1) (A) receptors.
Physicochemical Properties
| Molecular Formula | C12H16CLN3O2 | |
| Molecular Weight | 269.73 | |
| Exact Mass | 269.093 | |
| Elemental Analysis | C, 53.44; H, 5.98; Cl, 13.14; N, 15.58; O, 11.86 | |
| CAS # | 269718-83-4 | |
| Related CAS # | Pardoprunox; 269718-84-5 | |
| PubChem CID | 6918524 | |
| Appearance | Off-white to pink pale mauve solid powder | |
| LogP | 2.09 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 1 | |
| Heavy Atom Count | 18 | |
| Complexity | 302 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O=C1OC2=C(N3CCN(C)CC3)C=CC=C2N1.[H]Cl |
|
| InChi Key | NQRIKTDKFHAOKC-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C12H15N3O2.ClH/c1-14-5-7-15(8-6-14)10-4-2-3-9-11(10)17-12(16)13-9;/h2-4H,5-8H2,1H3,(H,13,16);1H | |
| Chemical Name | 7-(4-methylpiperazin-1-yl)-3H-1,3-benzoxazol-2-one;hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | 5-HT1A Receptor ( pEC50 = 6.3 ); D2 Receptor ( pEC50 = 8 ); D3 Receptor ( pEC50 = 9.2 nM ) | ||
| ln Vitro | Pardoprunox (SLV-308) hydrochloride has a 50% efficacy on forskolin-stimulated cAMP accumulation and functions as a strong but partial D2 receptor agonist (pEC50= 8.0 and pA2= 8.4). Pardoprunox hydrochloride antagonizes the dopamine-induced [(35)S]GTPgammaS binding (pA2=9.0) and functions as a partial agonist at human recombinant dopamine D3 receptors, inducing [(35)S]GTPgammaS binding with an intrinsic activity of 67%. On forskolin-induced cAMP accumulation at cloned human 5-HT1A receptors, pardoprunox hydrochloride functions as a full 5-HT1A receptor agonist, albeit with low potency (pEC50=6.3)[1]. | ||
| ln Vivo |
|
||
| Enzyme Assay | Pardoprunox also exhibits a lower affinity for binding to D4 (pKi = 7.8), α1-adrenergic (pKi = 7.8), α2-adrenergic (pKi = 7.4), and 5-HT7 receptors (pKi = 7.2). With a 50% efficacy on forskolin-stimulated cAMP accumulation, pardoprunox exerts a strong but partial D(2) receptor agonist (pEC50 = 8.0 and pA2 = 8.4). Pardoprunox inhibits the dopamine-induced induction of [35S]GTPgammaS binding (pA2 = 9.0) and functions as a partial agonist at human recombinant dopamine D3 receptors, where it induces [35S]GTPgammaS binding with an intrinsic activity of 67%. While cloned human 5-HT1A receptors are induced to accumulate cAMP by forskolin, pardoprunox functions as a full 5-HT1A receptor agonist with a low potency (pEC50 = 6.3). | ||
| Animal Protocol |
|
||
| References |
[1]. In vitro characterization of SLV308 (7-[4-methyl-1-piperazinyl]-2(3H)-benzoxazolone, monohydrochloride): a novel partial dopamine D2 and D3 receptor agonist and serotonin 5-HT1A receptor agonist. Synapse. 2006 Dec 15;60(8):599-608. [2]. An in vivo pharmacological evaluation of pardoprunox (SLV308)--a novel combined dopamine D(2)/D(3) receptor partial agonist and 5-HT(1A) receptor agonist with efficacy in experimental models of Parkinson's disease. Eur Neuropsychopharmacol . 2010 Aug;20(8):582-93. |
Solubility Data
| Solubility (In Vitro) | DMSO: 30~150 mg/mL (111.2~556.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 7.5 mg/mL (27.81 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 75.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 7.5 mg/mL (27.81 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 75.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 7.5 mg/mL (27.81 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 75.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7074 mL | 18.5371 mL | 37.0741 mL | |
| 5 mM | 0.7415 mL | 3.7074 mL | 7.4148 mL | |
| 10 mM | 0.3707 mL | 1.8537 mL | 3.7074 mL |