Physicochemical Properties
| Molecular Formula | C22H19F3N4O3 |
| Molecular Weight | 444.406475305557 |
| Exact Mass | 444.14 |
| CAS # | 2790567-82-5 |
| PubChem CID | 164536956 |
| Appearance | White to light yellow solid powder |
| LogP | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 32 |
| Complexity | 688 |
| Defined Atom Stereocenter Count | 1 |
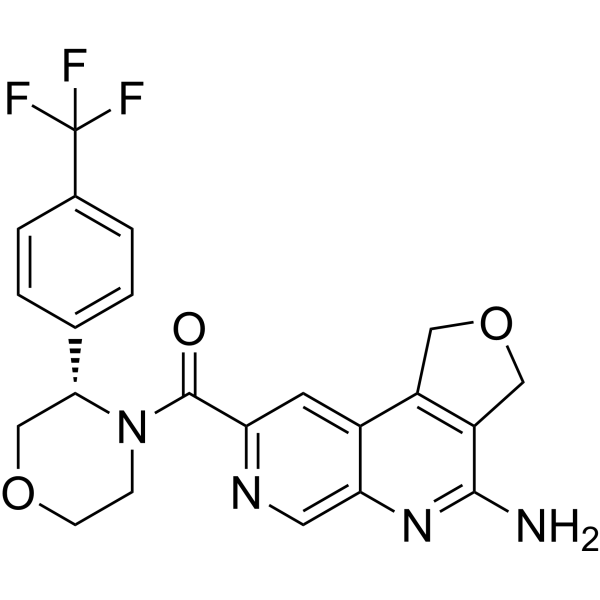
| SMILES | C1COC[C@@H](N1C(=O)C2=NC=C3C(=C2)C4=C(COC4)C(=N3)N)C5=CC=C(C=C5)C(F)(F)F |
| InChi Key | BFEBTMFPRJPBTK-LJQANCHMSA-N |
| InChi Code | InChI=1S/C22H19F3N4O3/c23-22(24,25)13-3-1-12(2-4-13)19-11-31-6-5-29(19)21(30)17-7-14-15-9-32-10-16(15)20(26)28-18(14)8-27-17/h1-4,7-8,19H,5-6,9-11H2,(H2,26,28)/t19-/m1/s1 |
| Chemical Name | (4-amino-1,3-dihydrofuro[3,4-c][1,7]naphthyridin-8-yl)-[(3S)-3-[4-(trifluoromethyl)phenyl]morpholin-4-yl]methanone |
| Synonyms | AMG193; AMG-193 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PRMT5 |
| ln Vitro | AMG 193 is a second-generation protein arginine methyltransferase 5 (PRMT5) inhibitor that targets the MTA-bound state of PRMT5 in methylthioadenosine phosphorylase (MTAP)-null tumors. PRMT5 is responsible for methylation and gene silencing of cell-essential proteins dysregulated in cancer and is partially inhibited in tumors harboring MTAP deletion, which occurs in ~15% of solid tumors[2]. |
| ln Vivo | First generation PRMT5 inhibitors were intolerable due to indiscriminate inhibition of PRMT5 leading to dose-limiting myelosuppression. In preclinical studies, AMG 193 demonstrated selective antitumor activity in MTAP-null models by further suppressing PRMT5 function while sparing normal function, thereby improving upon first generation molecules. We report the initial clinical results from dose-escalation in the ongoing first-in-human (FIH) study[2]. |
| Animal Protocol |
Methods: AMG193 was orally administered in continuous 28-day cycles to patients (pts) with advanced MTAP-null solid tumors. Dose escalation proceeded via a BLRM method. The primary objectives include safety, tolerability, and identification of the maximum tolerated dose (MTD). Secondary objectives include preliminary antitumor activity by investigator-assessed RECIST, pharmacokinetics (PK) and pharmacodynamic (PD) effects.[2] Results: As of August 8, 2023, 47 pts with MTAP-null cancer (PDAC n = 10; NSCLC n = 6; CCA = 5; MESO n = 3; others n = 23) were enrolled in seven escalating cohorts. Five pts had DLTs, and exploration continues per protocol to identify the MTD. The most common TRAEs were nausea (45%), fatigue (26%), decreased appetite (17%), and vomiting (17%). Preliminary PK analyses showed dose-proportional systemic exposure with a half-life of 7–11 hrs. Among 31 pts who had at least one postbaseline scan, there were 5 with confirmed PRs [PDAC (–100%), ovarian Sertoli-Leydig (–59%), RCC (–58%), esophageal (–46%), and gallbladder cancer (–63%), 1 each], 14 with stable disease (including 9 with some degree of tumor shrinkage), and 12 with disease progression. All PRs were ongoing at the data cutoff with treatment durations of 140–275 days. PD effects demonstrated dose-dependent reduction in serum total SDMA levels and complete PRMT5 inhibition was confirmed in five pts with on-treatment biopsies spanning multiple dose levels. Exploratory analysis of changes in variant allele frequency by ctDNA demonstrated rapid treatment effects that was predictive and correlated with response.[2] Conclusion: AMG 193 is an MTA-cooperative PRMT5 inhibitor designed to induce synthetic lethality in MTAP-null solid tumors while sparing hematologic toxicity. The initial results of the FIH study demonstrate proof-of-concept with encouraging signs of preliminary clinical activity without evidence of myelosuppression. Dose escalation continues to proceed to establish the MTD. AMG 193 has demonstrated promise as a potential new therapeutic for pts with tumors that have MTAP loss[2]. |
| References |
[1]. AMG 193 Effective in Multiple Tumor Types. Cancer Discov. 2023 Dec 12;13(12):2492. doi: 10.1158/2159-8290.CD-NB2023-0079. [2].Abstract PR006: Initial results from first-in-human study of AMG 193, an MTA-cooperative PRMT5 inhibitor, in biomarker-selected solid tumors. Mol Cancer Ther (2023) 22 (12_Supplement): PR006. https://doi.org/10.1158/1535-7163.TARG-23-PR006. [3]. Shon Booker, et al. Prmts inhibitors. WO2022132914A1. 2022-06-23. |
| Additional Infomation |
The second-generation PRMT5 inhibitor AMG 193 (Amgen) yielded partial responses in patients with a variety of tumor types while avoiding the toxicity associated with such first-generation agents. Reported at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, held October 11–15 in Boston, MA, the findings point to a possible treatment avenue for the 10% to 15% of patients who have MTAP-deleted solid tumors. “PRMT5 is responsible for methylation and gene silencing of cell-essential proteins dysregulated in cancer,” explained Jordi Rodón, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, who presented the findings. But broadly targeting PRMT5, as researchers had learned, caused serious myelosuppression and other intolerable effects, necessitating a different plan of attack. On the biology front, researchers knew that tumors with MTAP loss accumulate the metabolite MTA. “Interestingly,” Rodón continued, “MTA is a natural inhibitor of PRMT5, so tumors having MTAP loss accumulate MTA and have a partial inhibition of PRMT5. With chemical mastery, you can develop drugs that bind to PRMT5 only in the presence of MTA,” killing tumor cells while sparing healthy ones. View More
That's the thinking behind the new class of drugs called MTA-cooperative PRMT5 inhibitors, of which AMG 193 is one. Another is Mirati's MRTX1719. Boston-based Tango Therapeutics, which has two MTA-cooperative PRMT5 inhibitors in early-stage trials, Kraków, Poland's Ryvu Therapeutics, and Shanghai, China's Abbisko Therapeutics presented preclinical research on their respective agents at the Molecular Targets conference as well. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2502 mL | 11.2509 mL | 22.5017 mL | |
| 5 mM | 0.4500 mL | 2.2502 mL | 4.5003 mL | |
| 10 mM | 0.2250 mL | 1.1251 mL | 2.2502 mL |