Physicochemical Properties
| Molecular Formula | C20H26CLF3N6O3 |
| Molecular Weight | 490.9071 |
| Exact Mass | 490.17 |
| Elemental Analysis | C, 48.93; H, 5.34; Cl, 7.22; F, 11.61; N, 17.12; O, 9.78 |
| CAS # | 2253640-49-0 |
| PubChem CID | 137935722 |
| Appearance | Typically exists as solid at room temperature |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 33 |
| Complexity | 586 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | NCC1CCC(CNC2=NC(NCC3=C(C=CC=C3)OC(F)(F)F)=NC=C2[N+]([O-])=O)CC1.Cl |
| InChi Code | InChI=1S/C20H25F3N6O3.ClH/c21-20(22,23)32-17-4-2-1-3-15(17)11-26-19-27-12-16(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14;/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28);1H |
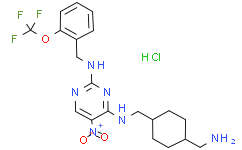
| Chemical Name | N4-((4-(aminomethyl)cyclohexyl)methyl)-5-nitro-N2-(2-(trifluoromethoxy)benzyl)pyrimidine-2,4-diamine hydrochloride |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PKCθ |
| ln Vitro | An uHTS campaign was performed to identify selective inhibitors of PKC-theta. Initial triaging of the hit set based on selectivity and historical analysis led to the identification of 2,4-diamino-5-nitropyrimidines as potent and selective PKC-theta inhibitors. A homology model and initial SAR is presented demonstrating that a 2-arylalkylamino substituent in conjunction with suitable 4-diamino substituent are essential for achieving selectivity over many kinases. Additional hit to lead profiling is presented on selected compounds[1]. |
| Enzyme Assay | To address the issue of selectivity we tested analogs in a panel of kinases and found them to be highly selective. Figure 4 shows selectivity data for the 38 representative analogs, with IC50 values for PKC-θ <0.5 μM, against a panel of 13 kinases representing data from 494 individual dose responses. Potencies increase upward from the bottom of the figure. Some trends could be visualized even amongst this highly selective set. The analogs where the linker was extended tended to show reduced selectivity (46–48) as did the o-Ph analog 25. The observed high selectivity of these analogs can be rationalized by the tight requirement for a properly positioned amino group to interact with Asp508 coupled with the 2-benzylamino substituent which controls specificity through its interactions at the glycine-rich loop, the specificity surface, and the hinge regions[1]. |
| Cell Assay | In addition to selectivity, other hit to lead criteria included activity in cells as well as acceptable drug-like properties. The compounds were also shown to be ATP competitive. Cellular activity was assessed by measuring inhibition of IL-2 production in human CD4+ T cells activated by costimulation with anti-CD3 and anti-CD28 mAbs. We also assessed representative compounds for inhibition of CYP’s, stability to human liver microsomes (HLM), and permeability in Caco-2 cells (Table 5). Although these compounds contain a nitro group which is potential structural alert, the overall profile was acceptable for further advancement in the hit to lead process[1]. |
| References | [1]. Discovery of potent and selective PKC-θ inhibitors. Bioorg. Med. Chem. Lett. 17(1), 225-230 (2007). |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0370 mL | 10.1852 mL | 20.3703 mL | |
| 5 mM | 0.4074 mL | 2.0370 mL | 4.0741 mL | |
| 10 mM | 0.2037 mL | 1.0185 mL | 2.0370 mL |