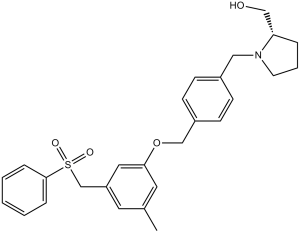
PF-543 (also called PF543; PF 543; Sphingosine Kinase 1 Inhibitor II) is a novel cell-permeable and sphingosine-competitive inhibitor of SphK1 with potential antitumor activity. It shows >100-fold selectivity for SphK1 over the SphK2 isoform and inhibits SphK1 with IC50s and Ki of 2.0 nM and 3.6 nM.
Physicochemical Properties
| Molecular Formula | C27H31NO4S | |
| Molecular Weight | 465.6 | |
| Exact Mass | 465.197 | |
| Elemental Analysis | C, 69.65; H, 6.71; N, 3.01; O, 13.74; S, 6.89 | |
| CAS # | 1415562-82-1 | |
| Related CAS # | PF-543 Citrate; 1415562-83-2; PF-543 hydrochloride; 1706522-79-3 | |
| PubChem CID | 66577038 | |
| Appearance | White to off-white Waxy semisolid | |
| Density | 1.2±0.1 g/cm3 | |
| Boiling Point | 666.0±55.0 °C at 760 mmHg | |
| Flash Point | 356.6±31.5 °C | |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C | |
| Index of Refraction | 1.610 | |
| LogP | 4.16 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 9 | |
| Heavy Atom Count | 33 | |
| Complexity | 679 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | CC1=CC(OCC2=CC=C(CN3[C@@H](CO)CCC3)C=C2)=CC(CS(C4=CC=CC=C4)(=O)=O)=C1 |
|
| InChi Key | NPUXORBZRBIOMQ-RUZDIDTESA-N | |
| InChi Code | InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | |
| Chemical Name | [(2R)-1-[[4-[[3-(benzenesulfonylmethyl)-5-methylphenoxy]methyl]phenyl]methyl]pyrrolidin-2-yl]methanol | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | SphK1 | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | The SphK enzyme assay, which uses a microfluidic capillary electrophoresis mobility-shift system to separate unreacted FITC-sphingosine substrate from FITC-S1P, is developed in a 384-well format. In summary, 1 μM FITC-sphingosine, 20 μM ATP, and 10 μM compound (with a final DMSO concentration of 2%), along with 3 nM SphK1–His6, are incubated for 1 hour in a 384-well Matrical MP-101-1-PP plate with a buffer that contains 100 mM Hepes (pH 7.4), 1 mM MgCl2, 0.01% Triton X-100, 10% glycerol, 100 μM sodium orthovanadate, and 1 mM DTT. The reaction mixtures (10 μL) are quenched by adding 20 μL of 30 mM EDTA and 0.15% Coating Reagent-3 in 100 mM Hepes. A small aliquot of each reaction, a few nanoliters, is then analyzed in the Caliper LabChip 3000 instrument at a downstream voltage of -1900 V, a sip time of 0.2 s, and a pressure of -1.5 psi (psi=6.9 kPa). The Caliper data are used to quantify the distinct peaks that appeared as phosphorylated fluorescent product and unphosphorylated fluorescent substrate. | ||
| Cell Assay | In 1483 head and neck cancer cell cultures that expressed high levels of SphK1 and produced S1P at an exceptionally high rate, pretreatment with PF-543 for one hour reduced endogenous S1P levels by ten times while increasing sphingosine levels proportionately. Significant inhibition of SphK1 BY pf-543 was observed. Specific inhibition of SphK1, however, did not affect the survival and proliferation of 1483 cell cultures, even though the cellular S1P/sphingosine rate changed dramatically. | ||
| Animal Protocol |
|
||
| References |
[1]. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem J. 2012 May 15;444(1):79-88. [2]. Effect of the sphingosine kinase 1 selective inhibitor, PF-543 on arterial and cardiac remodelling in a hypoxic model of pulmonary arterial hypertension. Cell Signal. 2016 Aug;28(8):946-55. [3]. Induction of autophagy by sphingosine kinase 1 inhibitor PF-543 in head and neck squamous cell carcinoma cells. Cell Death Discov. 2017 Aug 14;3:17047. |
||
| Additional Infomation | [(2R)-1-[[4-[[3-(benzenesulfonylmethyl)-5-methylphenoxy]methyl]phenyl]methyl]-2-pyrrolidinyl]methanol is a sulfonamide. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 5 mg/mL (10.74 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 5 mg/mL (10.74 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1478 mL | 10.7388 mL | 21.4777 mL | |
| 5 mM | 0.4296 mL | 2.1478 mL | 4.2955 mL | |
| 10 mM | 0.2148 mL | 1.0739 mL | 2.1478 mL |