PDM-2 (PDM2), a trans-resveratrol analog, is a novel selective, high-affinity aryl hydrocarbon receptor (AhR) antagonist with an Ki of 1.2±0.4 nM. This study investigated the in vitro protective effects of three derivatives of resveratrol, i.e., piceatannol, PDM2 and PDM11, compared with resveratrol as reference compound, against oxidation of linoleate micelles (10(-2)M) initiated by radiolysis-generated hydroxyl radicals. Lipid peroxidation was monitored by conjugated dienes (differential absorbance at 234nm), and by hydroperoxides (reverse phase HPLC with chemiluminescence detection). The higher the concentration of resveratrol or piceatannol (from 10(-5)M to 10(-4)M), the stronger the antioxidant ability. Piceatannol, with the presence of an additional hydroxyl group, showed a better antioxidant effect than resveratrol for a given concentration (competition with the fatty acid to scavenge lipid peroxyl radicals LOO), whereas PDM2 and PDM11, without any hydroxyl group, did not exhibit any significant protective effect. A lower limit for the LOO rate constant has been estimated for piceatannol (>/=1.4x10(5)M(-1)s(-1)) and for resveratrol (>/=0.3x10(5)M(-1)s(-1)).
Physicochemical Properties
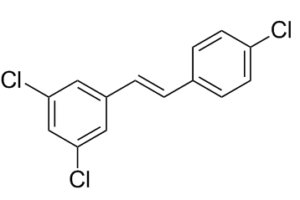
| Molecular Formula | C14H9CL3 | |
| Molecular Weight | 283.58000 | |
| Exact Mass | 281.98 | |
| Elemental Analysis | C, 59.30; H, 3.20; Cl, 37.50 | |
| CAS # | 688348-25-6 | |
| Related CAS # |
|
|
| PubChem CID | 9838722 | |
| Appearance | White to off-white solid powder | |
| LogP | 6.1 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 0 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 17 | |
| Complexity | 246 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | C1=CC(=CC=C1/C=C/C2=CC(=CC(=C2)Cl)Cl)Cl |
|
| InChi Key | JMYNPQVCVQVODQ-OWOJBTEDSA-N | |
| InChi Code | InChI=1S/C14H9Cl3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9H/b2-1+ | |
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | PDM2 (compound 4b) shows no affinity for the estrogen receptor (ER) and a Ki value of 1.2±0.4 nM for AhR, confirming that substituting chlorine for the hydroxyl group will prevent binding to the ER and greatly boost the affinity for AhR [1]. |
| References |
[1]. Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J Med Chem. 2005 Jan 13;48(1):287-91. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (8.82 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5263 mL | 17.6317 mL | 35.2634 mL | |
| 5 mM | 0.7053 mL | 3.5263 mL | 7.0527 mL | |
| 10 mM | 0.3526 mL | 1.7632 mL | 3.5263 mL |