Physicochemical Properties
| Molecular Formula | C8H12CLNO2 |
| Molecular Weight | 189.63 |
| Exact Mass | 189.055 |
| Elemental Analysis | C, 50.67; H, 6.38; Cl, 18.69; N, 7.39; O, 16.87 |
| CAS # | 770-05-8 |
| Related CAS # | Octopamine-d4 hydrochloride; 1219803-62-9 |
| PubChem CID | 440266 |
| Appearance | White to off-white solid powder |
| Boiling Point | 360.7ºC at 760 mmHg |
| Melting Point | ~170 °C (dec.)(lit.) |
| Flash Point | 172ºC |
| LogP | 1.886 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 11 |
| Complexity | 111 |
| Defined Atom Stereocenter Count | 1 |
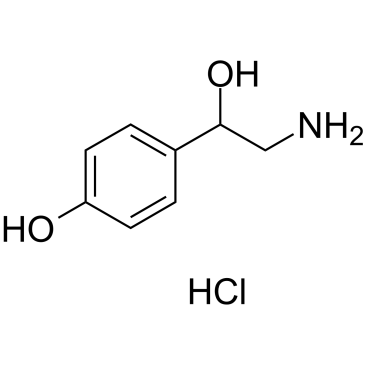
| SMILES | Cl[H].O([H])C([H])(C([H])([H])N([H])[H])C1C([H])=C([H])C(=C([H])C=1[H])O[H] |
| InChi Key | PUMZXCBVHLCWQG-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C8H11NO2.ClH/c9-5-8(11)6-1-3-7(10)4-2-6;/h1-4,8,10-11H,5,9H2;1H |
| Chemical Name | 4-(2-amino-1-hydroxyethyl)phenol;hydrochloride |
| Synonyms | Octopamine Hydrochloride; Octopamine Hydrochloride salt; Octopamine HCl; Octopamine HCl salt |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Human Endogenous Metabolite; α2-adrenergic receptor |
| ln Vitro | Octopamine is involved in a number of physiological processes: (a) it acts as a neuromodulator, controlling the desensitization of sensory inputs, arousal, initiation, and maintenance of complex behaviors like learning and memory; (b) it acts as a neurotransmitter, controlling the activity of endocrine glands; and (c) it acts as a neurohormone, causing the mobilization of fats and carbohydrates. In order to cause its effects, octopamine binds to particular proteins that are members of the G protein-coupled receptor superfamily and have seven transmembrane domains in common structurally[1]. |
| References |
[1]. Octopamine-mediated neuromodulation of insect senses. Neurochem Res. 2007;32(9):1511-1529. [2]. Octopamine in invertebrates. Prog Neurobiol. 1999;59(5):533-561. [3]. Octopamine. Nature. 1977;265(5594):501-504. |
| Additional Infomation |
(R)-octopamine is an octopamine. It is an enantiomer of a (S)-octopamine. p-Hydroxyphenylethanolamine has been reported in Aplysia californica, Fascaplysinopsis reticulata, and other organisms with data available. An alpha-adrenergic sympathomimetic amine, biosynthesized from tyramine in the CNS and platelets and also in invertebrate nervous systems. It is used to treat hypotension and as a cardiotonic. The natural D(-) form is more potent than the L(+) form in producing cardiovascular adrenergic responses. It is also a neurotransmitter in some invertebrates. See also: Octopamine (annotation moved to). |
Solubility Data
| Solubility (In Vitro) |
DMSO: ≥ 100 mg/mL (~527.3 mM) H2O: ≥ 50 mg/mL (~263.7 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 100 mg/mL (527.31 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.2734 mL | 26.3671 mL | 52.7343 mL | |
| 5 mM | 1.0547 mL | 5.2734 mL | 10.5469 mL | |
| 10 mM | 0.5273 mL | 2.6367 mL | 5.2734 mL |