ODQ is a novel, potent and selective inhibitor of soluble guanylyl cyclase (sGC). ODQ and NO have a competitive binding. cGMP is produced by the nitric oxide receptor known as soluble guanylyl cyclase (sGC). Less is known about this second messenger molecule's effects in tumor cells, despite its established roles in cellular physiology. In LNCaP cells, ODQ's activity did not resemble sGC inhibition. Prostate cancer cells are inhibited in their growth and migration, and their ability to die is enhanced by ODQ, all of which occur independently of the compound's impact on GMP levels.
Physicochemical Properties
| Molecular Formula | C9H5N3O2 | |
| Molecular Weight | 187.15 | |
| Exact Mass | 187.038 | |
| Elemental Analysis | C, 57.76; H, 2.69; N, 22.45; O, 17.10 | |
| CAS # | 41443-28-1 | |
| Related CAS # |
|
|
| PubChem CID | 1456 | |
| Appearance | Off-white to yellow solid powder | |
| Density | 1.6±0.1 g/cm3 | |
| Boiling Point | 321.3±25.0 °C at 760 mmHg | |
| Melting Point | 160-170 °C | |
| Flash Point | 148.1±23.2 °C | |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C | |
| Index of Refraction | 1.781 | |
| LogP | 0.28 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 0 | |
| Heavy Atom Count | 14 | |
| Complexity | 337 | |
| Defined Atom Stereocenter Count | 0 | |
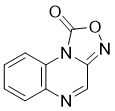
| SMILES | O1C(N2C(C([H])=NC3=C([H])C([H])=C([H])C([H])=C23)=N1)=O |
|
| InChi Key | LZMHWZHOZLVYDL-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C9H5N3O2/c13-9-12-7-4-2-1-3-6(7)10-5-8(12)11-14-9/h1-5H | |
| Chemical Name | [1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | sGC/soluble guanylyl cyclase (nitric oxide-activated enzyme) |
| ln Vitro | Rat cardiomyoblasts are exposed to H2O2 in vitro for 4 hours, which reduces mitochondrial respiration because it produces hydroxyl radicals. ODQ pretreatment of the cells does not prevent this cell damage. Additionally, superoxide anions are not scavenged by ODQ. [1] |
| ln Vivo |
Rats pretreated with ODQ have reduced lung damage, hepatocellular injury, and renal dysfunction brought on by lipoteichoic acid/peptidoglycan or lipopolysaccharide.[1] In vivo, administration of lipoteichoic acid/peptidoglycan or lipopolysaccharide resulted within 6 hrs in hypotension, acute renal dysfunction, hepatocellular injury, and lung injury. Pretreatment of rats with ODQ attenuated the renal dysfunction, lung injury, and hepatocellular injury caused by lipoteichoic acid/peptidoglycan or lipopolysaccharide. In vitro, administration of H2O2 (for 4 hrs) to rat cardiomyoblasts decreased mitochondrial respiration attributable to generation of hydroxyl radicals. Pretreatment of cells with ODQ did not abolish this cell injury. In addition, ODQ did not scavenge superoxide anions. Conclusions: These results imply that ODQ, an inhibitor of guanylate cyclase, reduces the multiple organ injury and dysfunction caused by wall fragments of Gram-positive or Gram-negative bacteria in the anesthetized rat. The observed protective effects of ODQ are not attributable to the ability of ODQ to reduce the formation or the effects of superoxide anions or hydroxyl radicals[1]. |
| Enzyme Assay |
Evaluation of the Effects of ODQ on the Generation of Superoxide Anions.[1] We used a hypoxanthine/xanthine-oxidase assay to generate superoxide anions as previously described. Briefly, an aqueous xanthine-oxidase solution (2 units/mL) and hypoxanthine (0.7 mmol/L) were taken as substrates. Luminol-bound albumin was prepared by stirring equal weights (10 mg/mL) of bovine serum albumin and luminol in phosphate-buffered saline overnight. The solution then was warmed to 40°C to increase the solubility of luminol. The solution was filtered through a 0.22-μm filter to obtain a clear solution, which was frozen and stored. DMSO was used to stabilize the chemiluminescence according to Trevithick et al. The solution was incubated with ODQ (0.1 μM to 1 mM), and xanthine-oxidase/hypoxanthine was added just before the measurement period. The chemiluminescence was recorded every 13 secs with a commercially available counter. All experiments were carried out in duplicate of n = 4 observations. |
| Cell Assay |
H2O2 is a helpful instrument for researching how various organs are affected by reactive oxygen species. It can easily permeate through cell membranes and, in the presence of transition metals, undergo the Fenton reaction at intracellular locations to transform into the toxic hydroxyl radical. Preincubation of the cells was conducted for two hours at 37°C using ODQ (0.1 mM to 1 mM), saline, or DMSO (media containing 10% DMSO). Following a 4-hour exposure to media or H2O2 (1 mM) at 37°C, the cells' degree of damage is measured. Every experiment is run twice using n = 4 observations. Evaluation of the Effects of ODQ on the Cell Injury Caused by H2O2 in Rat Cardio-myoblasts: Experimental Design.[1] H2O2 is a useful tool for studying the effects of reactive oxygen species on different organs. It can readily diffuse across cell membranes (36) and can be converted into the toxic hydroxyl radical at intracellular sites via the Fenton reaction in the presence of transition metals. Cells were preincubated (2 hrs, 37°C) with a) ODQ(0.1 μM to 1 mM), b) saline, or c) DMSO (media containing 10% DMSO). The cells then were exposed to a) media or b) H2O2 (1 mM for 4 hrs at 37°C) after which time cell injury was assessed (see previous description). All experiments were carried out in duplicate of n = 4 observations. |
| Animal Protocol |
Anesthetized, male Wistar rats 2 mg/kg I.p. For the in vivo portion of the study, after surgical preparation, anesthetized rats were observed for 6 hrs. All rats were pretreated and received an intravenous infusion of saline (1.5 mL·kg−1·hr−1), which was maintained throughout the experiment. The rats were assigned to nine groups. Group 1 contained control rats (sham) subjected to 2 mL/kg saline intraperitoneally, 2 hrs before the experiment (n = 7). Group 2 contained control rats (sham) that received 2 mg/kg ODQ intraperitoneally, 2 hrs before the experiment (n = 9). Group 3 contained control rats (sham) that received 2 mL/kg dimethyl sulfoxide, 30% v/v in saline intraperitoneally, as a vehicle for ODQ, 2 hrs before the experiment (n = 6). In group 4 rats, Gram-positive shock was induced by coadministration of lipoteichoic acid (3 mg/kg intravenously) and peptidoglycan (10 mg/kg intravenously) (n = 10). In group 5, rats were pretreated with ODQ (as described previously) before lipoteichoic acid/peptidoglycan (n = 9). In group 6, rats were pretreated with dimethyl sulfoxide (as de- scribed previously) before lipoteichoic acid/peptidoglycan (n = 7). In group 7, Gram-negative shock was induced by lipopolysaccharide (6 mg/kg intravenously) (n = 11). In group 8, rats were pretreated with ODQ (as described previously) before lipopolysaccharide (n = 8). In group 9, rats were pretreated with dimethyl sulfoxide (as described previously) before lipopolysaccharide (n = 8).[1] Evaluation of the Effects of ODQon Circulatory Failure and Multiple Organ Dysfunction Syndrome: Experimental Design.[1] Nine experimental groups were studied. After an injection of drugs (saline, 2 mL/kg intraperitoneally; ODQ, 2 mg/kg intraperitoneally; or dimethyl sulfoxide [DMSO] as vehicle for ODQ, 2 mL/kg DMSO 30% v/v in saline intraperitoneally), rats were connected to an infusion of saline (1.5 mL·kg−1·hr−1 intravenously), which was maintained throughout the experiment. The nine groups were as follows: Control rats were treated with saline 2 hrs before the experiment (sham saline, n = 6). Control rats were treated with ODQ 2 hrs before the experiment (sham ODQ, n = 7). Control rats were treated with vehicle for ODQ 2 hrs before the experiment (sham DMSO, n = 6). Rats were treated with saline 2 hrs before they were subjected to Gram-positive shock: LTA (Staphylococcus aureus, 3 mg/kg intravenously) was given over 1 min followed by PepG (S. aureus, 10 mg/kg intravenously) over 15 mins (LTA/PepG, n = 8). Rats were treated with ODQ 2 hrs before they were subjected to Gram-positive shock (as described previously) (ODQ + LTA/PepG, n = 8). Rats were treated with vehicle for ODQ (DMSO, as described previously) 2 hrs before they were subjected to Gram-positive shock (as described previously) (DMSO + LTA/PepG, n = 6). Rats were treated with saline 2 hrs before they were subjected to Gram-negative shock: LPS (Escherichia coli, 6 mg/kg intravenously) was given over 15 mins (LPS, n = 8). Rats were treated with ODQ 2 hrs before they were subjected to Gram-negative shock (as described previously; ODQ + LPS, n = 8). Rats were treated with vehicle for ODQ (DMSO) 2 hrs before they were subjected to Gram-negative shock (as described previously; DMSO + LPS, n = 6).[1] |
| References |
[1]. Crit Care Med . 2001 Aug;29(8):1599-608. |
| Additional Infomation | 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one is a member of the class of oxadiazoloquinoxalines that is 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline substituted at position 1 by an oxo group. It has a role as an EC 4.6.1.2 (guanylate cyclase) inhibitor. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5 mg/mL (26.72 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.3433 mL | 26.7165 mL | 53.4331 mL | |
| 5 mM | 1.0687 mL | 5.3433 mL | 10.6866 mL | |
| 10 mM | 0.5343 mL | 2.6717 mL | 5.3433 mL |