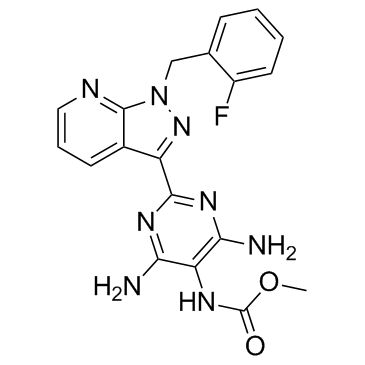
Nelociguat (also known as BAY60-4552) is a nitric oxide sensitive soluble guanylate cyclase stimulator (sGC stimulator). In the nitric oxide (NO) signaling pathway, soluble guanylate cyclase (sGC) is a crucial enzyme. Cyclic guanosine monophosphate (cGMP), which boosts vasodilation and inhibits smooth muscle proliferation, leukocyte recruitment, platelet aggregation, and vascular remodelling through a variety of downstream mechanisms, is synthesized by sGC upon binding of NO to its prosthetic haem group.
Physicochemical Properties
| Molecular Formula | C19H17FN8O2 |
| Molecular Weight | 408.38908 |
| Exact Mass | 408.145 |
| Elemental Analysis | C, 55.88; H, 4.20; F, 4.65; N, 27.44; O, 7.84 |
| CAS # | 625115-52-8 |
| PubChem CID | 11690019 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 533.3±50.0 °C at 760 mmHg |
| Flash Point | 276.3±30.1 °C |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.748 |
| LogP | -0.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 30 |
| Complexity | 589 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(OC)NC1=C(N)N=C(C2=NN(CC3=CC=CC=C3F)C4=NC=CC=C42)N=C1N |
| InChi Key | FTQHGWIXJSSWOY-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C19H17FN8O2/c1-30-19(29)24-14-15(21)25-17(26-16(14)22)13-11-6-4-8-23-18(11)28(27-13)9-10-5-2-3-7-12(10)20/h2-8H,9H2,1H3,(H,24,29)(H4,21,22,25,26) |
| Chemical Name | methyl N-[4,6-diamino-2-[1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]carbamate |
| Synonyms | Nelociguat; BAY604552; BAY-604552; BAY 604552; BAY60-4552; BAY 60-4552; BAY-60-4552 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | In the nitric oxide (NO) signaling pathway, soluble guanylate cyclase (sGC) is an essential enzyme[1]. In addition to cytochrome P450 isoenzymes 3A4 (CYP3A4), CYP2C8, and CYP2J2, riciguat is also metabolized to BAY60-4552 via CYP1A1, which is found in the liver and lungs[2]. |
| ln Vivo | GSK2181236A and BAY 60-4552 offer some protection against end-organ damage brought on by hypertension. A small dose of BAY 60-4552 reduces urine output and increases survival in rats that are prone to spontaneous hypertension and stroke. In addition to lowering microalbuminuria and reducing urine output, a high dose also attenuates the rise in mean arterial pressure. The survival rates of 46 and 69% are increased by BAY 60-4552 at doses of 0.3 and 3 mg/kg/day. Urine output is dose-dependently decreased to 79±11 and 56±10 mL/day after seven weeks of treatment with BAY 60-4552 (0.3 and 3.0 mg/kg/day)[1]. Vardenafil and BAY 60-4552 have synergistic beneficial effects that may be able to save patients who are not responding well to PDE5 inhibitor treatment following radical prostatectomy[3]. |
| Animal Protocol | Rats: Oral gavage of rats is performed two hours before ischemia with vehicle (0.5% HPMC, 5% DMSO, and 0.1% Tween 80; 10 mL/kg; n=14), GSK2181236A (0.1 or 1.0 mg/kg; n=11–14), or BAY 60-4552 (0.3 or 3.0 mg/kg; n=10–12). Both when ischemia first occurs and 24 hours after reperfusion, blood is drawn. For analysis, plasma is obtained[1]. |
| References |
[1]. Comparison of soluble guanylate cyclase stimulators and activators in models of cardiovascular disease associated with oxidative stress. Front Pharmacol. 2012 Jul 5;3:128. [2]. Pharmacokinetics of the Soluble Guanylate Cyclase Stimulator Riociguat in Healthy Young Chinese Male Non-Smokers and Smokers: Results of a Randomized, Double-Blind, Placebo-Controlled Study. Clin Pharmacokinet. 2016 May;55(5):615-24. [3]. Combination of BAY 60-4552 and vardenafil exerts proerectile facilitator effects in rats with cavernous nerve injury: a proof of concept study for the treatment of phosphodiesterase type 5 inhibitor failure. Eur Urol. 2011 Nov;60(5):1020-6. |
| Additional Infomation | Nelociguat is a member of the class of pyrazolopyridines that is 1H-pyrazolo[3,4-b]pyridine which is substituted by a 2-fluorobenzyl and 4,6-diamino-5-[(methoxycarbonyl)amino]pyrimidin-2-yl groups at positions 1 and 3, respectively. It is an active metabolite of riociguat and a soluble guanylate cyclase stimulator developed by Bayer for the treatment of erectile dysfunction and heart failure. It has a role as a soluble guanylate cyclase activator, an antihypertensive agent, a drug metabolite and a vasodilator agent. It is a pyrazolopyridine, a member of monofluorobenzenes, a carbamate ester and an aminopyrimidine. |
Solubility Data
| Solubility (In Vitro) | DMSO: < 1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (5.09 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (5.09 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4486 mL | 12.2432 mL | 24.4864 mL | |
| 5 mM | 0.4897 mL | 2.4486 mL | 4.8973 mL | |
| 10 mM | 0.2449 mL | 1.2243 mL | 2.4486 mL |