Physicochemical Properties
| Molecular Formula | C15H20CL2N6O |
| Molecular Weight | 371.26 |
| Exact Mass | 370.107 |
| Elemental Analysis | C, 48.53; H, 5.43; Cl, 19.10; N, 22.64; O, 4.31 |
| CAS # | 207556-62-5 |
| Related CAS # | NVP-DPP728;247016-69-9 |
| PubChem CID | 9799555 |
| Appearance | Typically exists as solid at room temperature |
| LogP | 1.824 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 24 |
| Complexity | 475 |
| Defined Atom Stereocenter Count | 1 |
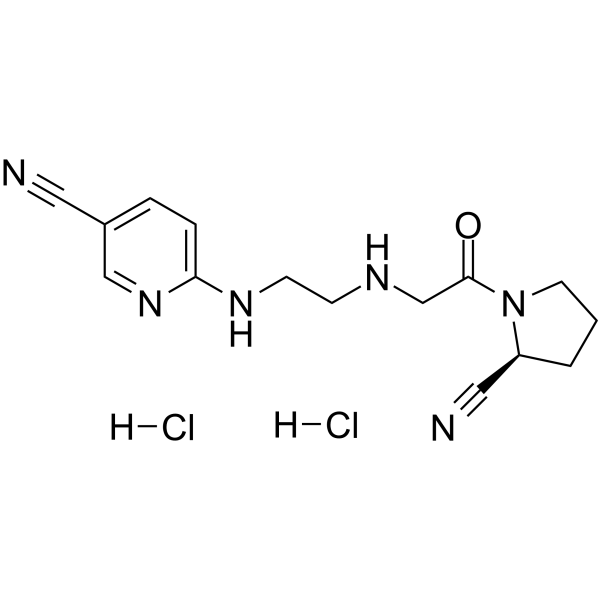
| SMILES | C1C[C@@H](C#N)N(C1)C(=O)CNCCN=C2C=CC(=CN2)C#N.Cl.Cl |
| InChi Key | VNACOBVZDCLAEV-GXKRWWSZSA-N |
| InChi Code | InChI=1S/C15H18N6O.2ClH/c16-8-12-3-4-14(20-10-12)19-6-5-18-11-15(22)21-7-1-2-13(21)9-17;;/h3-4,10,13,18H,1-2,5-7,11H2,(H,19,20);2*1H/t13-;;/m0../s1 |
| Chemical Name | 6-[2-[[2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl]amino]ethylamino]pyridine-3-carbonitrile;dihydrochloride |
| Synonyms | NVP DPP 728 DIHYDROCHLORIDE; PWKTKAMJUR; NVP-DPP-728 dihydrochloride; dpp-728; UNII-PWKTKAMJUR; DPP 728; NVP-DPP728 (dihydrochloride); |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Ki: 11 nM (DPP-IV) [1] |
| ln Vitro | A variety of proline-cleaving proteases and human and rat plasma DPP-IV are inhibited by NVPDPP728 with >15 000-fold selectivity when compared to DPP-II (IC50s: 5-10 nM)[1]. |
| ln Vivo | NVP-DPP728 (3.72 mg/kg; po) increases insulin secretion and glucose tolerance while inhibiting DPP-IV, most likely via amplifying the effects of endogenous GLP-1[2]. |
| Enzyme Assay | Inhibition of dipeptidyl peptidase IV (DPP-IV) has been proposed recently as a therapeutic approach to the treatment of type 2 diabetes. N-Substituted-glycyl-2-cyanopyrrolidide compounds, typified by NVP-DPP728 (1-[[[2-[(5-cyanopyridin-2-yl)amino]ethyl]amino]acetyl]-2-cyano-(S )-p yrrolidine), inhibit degradation of glucagon-like peptide-1 (GLP-1) and thereby potentiate insulin release in response to glucose-containing meals. In the present study NVP-DPP728 was found to inhibit human DPP-IV amidolytic activity with a K(i) of 11 nM, a k(on) value of 1.3 x 10(5) M(-)(1) s(-)(1), and a k(off) of 1.3 x 10(-)(3) s(-)(1). Purified bovine kidney DPP-IV bound 1 mol/mol [(14)C]-NVP-DPP728 with high affinity (12 nM K(d)). The dissociation constant, k(off), was 1.0 x 10(-)(3) and 1.6 x 10(-)(3) s(-)(1) in the presence of 0 and 200 microM H-Gly-Pro-AMC, respectively (dissociation t(1/2) approximately 10 min). Through kinetic evaluation of DPP-IV inhibition by the D-antipode, des-cyano, and amide analogues of NVP-DPP728, it was determined that the nitrile functionality at the 2-pyrrolidine position is required, in the L-configuration, for maximal activity (K(i) of 11 nM vs K(i) values of 5.6 to >300 microM for the other analogues tested). Surprisingly, it was found that the D-antipode, despite being approximately 500-fold less potent than NVP-DPP728, displayed identical dissociation kinetics (k(off) of 1.5 x 10(-)(3) s(-)(1)). NVP-DPP728 inhibited DPP-IV in a manner consistent with a two-step inhibition mechanism. Taken together, these data suggest that NVP-DPP728 inhibits DPP-IV through formation of a novel, reversible, nitrile-dependent complex with transition state characteristics[1]. |
| Animal Protocol |
Animal/Disease Models: Obese (fa/fa) and lean ( FA/?) Zucker rats[2] Doses: 3.72 mg/kg Route of Administration: Oral administration Experimental Results: Led to inhibition of plasma DPP-IV activity. An oral glucose tolerance test was done in lean and obese male Zucker rats while plasma DPP-IV was inhibited by the specific and selective inhibitor NVP-DPP728 given orally. Results: Inhibition of DPP-IV resulted in a significantly amplified early phase of the insulin response to an oral glucose load in obese fa/fa rats and restoration of glucose excursions to normal. In contrast, DPP-IV inhibition produced only minor effects in lean FA/? rats. Inactivation of GLP-1 (7-36) amide was completely prevented by DPP-IV inhibition suggesting that the effects of this compound on oral glucose tolerance are mediated by increased circulating concentrations of GLP-1 (7-36) amide. Reduced gastric emptying, as monitored by paracetamol appearance in the circulation after an oral bolus, did not appear to have contributed to the reduced glucose excursion. Conclusion/interpretation: It is concluded that NVP-DPP728 inhibits DPP-IV and improves insulin secretion and glucose tolerance, probably through augmentation of the effects of endogenous GLP-1. The improvement observed in prandial glucose homeostasis during DPP-IV inhibition suggests that inhibition of this enzyme is a promising treatment for Type II diabetes. [Diabetologia (1999) 42: 1324-1331] |
| References |
[1]. NVP-DPP728 (1-[[[2-[(5-cyanopyridin-2-yl)amino]ethyl]amino]acetyl]-2-cyano-(S)- pyrrolidine), a slow-binding inhibitor of dipeptidyl peptidase IV. Biochemistry. 1999 Sep 7;38(36):11597-603. [2]. Inhibition of dipeptidyl peptidase IV with NVP-DPP728 increases plasma GLP-1 (7-36 amide) concentrations and improves oral glucose tolerance in obese Zucker rats. Diabetologia. 1999 Nov;42(11):1324-31. |
| Additional Infomation | Aims/hypothesis: The potent incretin hormone glucagon-like peptide 1 (GLP-1) plays a pivotal role in prandial insulin secretion. In the circulation GLP-1 (7-36) amide is, however, rapidly (t(1/2):1-2 min) inactivated by the protease dipeptidyl peptidase IV (DPP-IV). We therefore investigated whether DPP-IV inhibition is a feasible approach to improve glucose homeostasis in insulin resistant, glucose intolerant fatty Zucker rats, a model of mild Type II (non-insulin-dependent) diabetes mellitus.[2] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6935 mL | 13.4677 mL | 26.9353 mL | |
| 5 mM | 0.5387 mL | 2.6935 mL | 5.3871 mL | |
| 10 mM | 0.2694 mL | 1.3468 mL | 2.6935 mL |