N6022 is a selective, first-in-class and reversible inhibitor of S-nitrosoglutathione reductase (GSNOR) with IC50 of 8 nM and Ki of 2.5 nM. Inhibition of GSNOR causes the accumulation of GSNO which acts as a vasodilator and anti-inflammatory factor. N6022 presents an IC50 value of 8nM in the GSNO reduction assay and 32nM in the HMGSH oxidation assay. The Ki values are 2.5nM and 3.1nM, respectively. N6022 is selective against GSNOR over other human ADH enzymes. The IC50 values are 21μM, 67μM and 0.5μM for ADH IB, ADH II and ADH IV, respectively.
Physicochemical Properties
| Molecular Formula | C24H22N4O3 | |
| Molecular Weight | 414.46 | |
| Exact Mass | 414.169 | |
| Elemental Analysis | C, 69.55; H, 5.35; N, 13.52; O, 11.58 | |
| CAS # | 1208315-24-5 | |
| Related CAS # |
|
|
| PubChem CID | 44623946 | |
| Appearance | white to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 662.7±55.0 °C at 760 mmHg | |
| Flash Point | 354.6±31.5 °C | |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C | |
| Index of Refraction | 1.664 | |
| LogP | 3.35 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 31 | |
| Complexity | 636 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | OC(CCC1=CC=C(N1C2=CC=C(C(N)=O)C=C2C)C(C=C3)=CC=C3N4C=CN=C4)=O |
|
| InChi Key | YVPGZQLRPAGKLA-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C24H22N4O3/c1-16-14-18(24(25)31)4-9-21(16)28-20(8-11-23(29)30)7-10-22(28)17-2-5-19(6-3-17)27-13-12-26-15-27/h2-7,9-10,12-15H,8,11H2,1H3,(H2,25,31)(H,29,30) | |
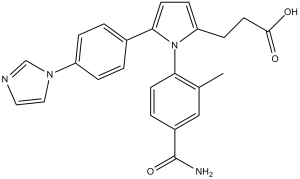
| Chemical Name | 3-(5-(4-(1H-imidazol-1-yl)phenyl)-1-(4-carbamoyl-2-methylphenyl)-1H-pyrrol-2-yl)propanoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | N6022 binds to rat plasma proteins in a concentration-dependent manner. N6022 has a higher impact on ATP than GSH[1] even at lower pharmacological concentrations (20 μM). With an IC50 of 8 nM and a Ki of 2.5 nM, N6022 functions as a competitive inhibitor when it binds in the GSNO substrate binding pocket. When it comes to cofactors NAD+ and NADH, N6022 is not competitive [2]. | ||
| ln Vivo | Rats given N6022 (50 mg/kg) showed a modest increase in the incidence of granulomas. N6022 has been found in serum at quantities as high as 5 mg/mL[1]. | ||
| Animal Protocol |
|
||
| References |
[1]. Structure-activity relationships of pyrrole based S-nitrosoglutathione reductase inhibitors: pyrrole regioisomers and propionic acid replacement. Bioorg Med Chem Lett. 2011 Jun 15;21(12):3671-5. [2]. A nonclinical safety and pharmacokinetic evaluation of N6022: a first-in-class S-nitrosoglutathione reductase inhibitor for the treatment of asthma. Regul Toxicol Pharmacol. 2012 Feb;62(1):115-24. [3]. Mechanism of inhibition for N6022, a first-in-class drug targeting S-nitrosoglutathione reductase. Biochemistry. 2012 Mar 13;51(10):2157-68. [4]. Methods of treating respiratory disorders. Patent. US 20170209419 A1. |
||
| Additional Infomation | N6022 has been used in trials studying the treatment of Asthma and Cystic Fibrosis. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.03 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.03 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4128 mL | 12.0639 mL | 24.1278 mL | |
| 5 mM | 0.4826 mL | 2.4128 mL | 4.8256 mL | |
| 10 mM | 0.2413 mL | 1.2064 mL | 2.4128 mL |