Physicochemical Properties
| Molecular Formula | C10H14N4O4 |
| Molecular Weight | 254.24 |
| Exact Mass | 254.102 |
| CAS # | 866363-70-4 |
| PubChem CID | 11594035 |
| Appearance | Colorless to light yellow liquid |
| LogP | 0.854 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 18 |
| Complexity | 373 |
| Defined Atom Stereocenter Count | 0 |
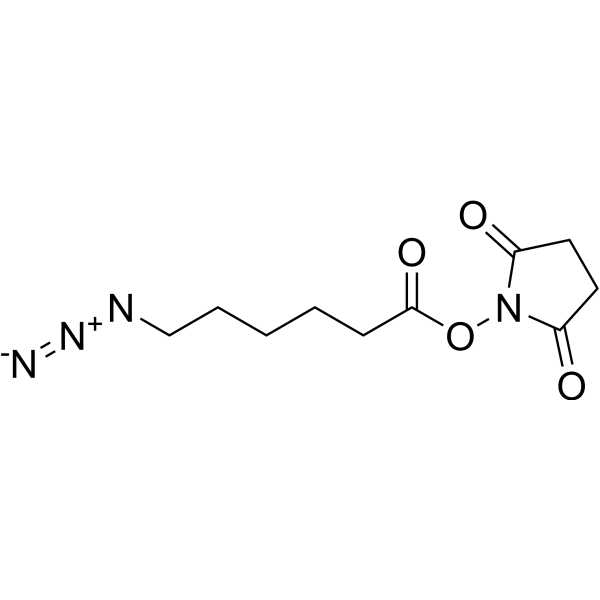
| SMILES | N(CCCCCC(ON1C(=O)CCC1=O)=O)=[N+]=[N-] |
| InChi Key | FVWGCTQOFSJEDB-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C10H14N4O4/c11-13-12-7-3-1-2-4-10(17)18-14-8(15)5-6-9(14)16/h1-7H2 |
| Chemical Name | (2,5-dioxopyrrolidin-1-yl) 6-azidohexanoate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Non-cleavable Linker |
| References |
[1]. Pyrrolobenzodiazepine Dimer Antibody-Drug Conjugates: Synthesis and Evaluation of Noncleavable Drug-Linkers. J Med Chem. 2017 Dec 14;60(23):9490-9507. |
Solubility Data
| Solubility (In Vitro) | DMSO : 100 mg/mL (393.33 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.83 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (9.83 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (9.83 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9333 mL | 19.6665 mL | 39.3329 mL | |
| 5 mM | 0.7867 mL | 3.9333 mL | 7.8666 mL | |
| 10 mM | 0.3933 mL | 1.9666 mL | 3.9333 mL |