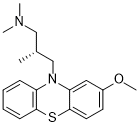
Methotrimeprazine (Levomepromazine) is a potent phenothiazine-based neuroleptic drug. It can be used to treat nausea and vomiting in palliative care settings and is orally bioavailable. Levomepromazine acts as an antagonist at several different neurotransmitter receptor sites, such as those for histamine, dopamine, cholinergic, and serotonin.
Physicochemical Properties
| Molecular Formula | C19H24N2OS |
| Molecular Weight | 328.47 |
| Exact Mass | 328.161 |
| Elemental Analysis | C, 69.47; H, 7.36; N, 8.53; O, 4.87; S, 9.76 |
| CAS # | 60-99-1 |
| Related CAS # | 7104-38-3; 1236-99-3 (HCl); 60-99-1 |
| PubChem CID | 72287 |
| Appearance | White to off-white solid powder |
| Density | 1.125g/cm3 |
| Boiling Point | 468ºC at 760 mmHg |
| Melting Point | 117°C |
| Flash Point | 236.8ºC |
| Index of Refraction | 1.594 |
| LogP | 4.56 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 23 |
| Complexity | 378 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | C[C@H](CN(C)C)CN1C2=CC=CC=C2SC3=C1C=C(C=C3)OC |
| InChi Key | VRQVVMDWGGWHTJ-CQSZACIVSA-N |
| InChi Code | InChI=1S/C19H24N2OS/c1-14(12-20(2)3)13-21-16-7-5-6-8-18(16)23-19-10-9-15(22-4)11-17(19)21/h5-11,14H,12-13H2,1-4H3/t14-/m1/s1 |
| Chemical Name | (2R)-3-(2-methoxyphenothiazin-10-yl)-N,N,2-trimethylpropan-1-amine |
| Synonyms | Nozinan; Nosinan; Levomepromazine; Methoxytrimeprazine; mepromazine; Methotrimeprazine; Milezin; Minozinan; Neozine |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Methotrimeprazine has an incomplete oral bioavailability, because it undergoes considerable first-pass-metabolism in the liver. Oral bioavailability is approximately 50 to 60%. Metabolism / Metabolites Hepatic. Methotrimeprazine is metabolized in the liver and degraded to a sulfoxid-, a glucuronid- and a demethyl-moiety. Hepatic. Methotrimeprazine is metabolized in the liver and degraded to a sulfoxid-, a glucuronid- and a demethyl-moiety. Half Life: Approximately 20 hours. Biological Half-Life Approximately 20 hours. |
| Toxicity/Toxicokinetics |
Toxicity Summary Methotrimeprazine's antipsychotic effect is largely due to its antagonism of dopamine receptors in the brain. In addition, its binding to 5HT2 receptors may also play a role. Methotrimeprazine exerts its actions through a central adrenergic-blocking, a dopamine-blocking, a serotonin-blocking, and a anticholinergic blocking. |
| References |
[1]. Levomepromazine for nausea and vomiting in palliative care. Cochrane Database Syst Rev. 2015 Nov 2;(11):CD009420. [2]. Levomepromazine for schizophrenia. Cochrane Database Syst Rev. 2010 Oct 6;(10):CD007779. |
| Additional Infomation |
Methotrimeprazine is a member of the class of phenothiazines that is 10H-phenothiazine substituted by a (2R)-3-(dimethylamino)-2-methylpropyl group and a methoxy group at positions 10 and 2 respectively. It has a role as a phenothiazine antipsychotic drug, a dopaminergic antagonist, a serotonergic antagonist, a cholinergic antagonist, a non-narcotic analgesic, an EC 3.4.21.26 (prolyl oligopeptidase) inhibitor and an anticoronaviral agent. It is a member of phenothiazines and a tertiary amine. It derives from a hydride of a 10H-phenothiazine. A phenothiazine with pharmacological activity similar to that of both chlorpromazine and promethazine. It has the histamine-antagonist properties of the antihistamines together with central nervous system effects resembling those of chlorpromazine. (From Martindale, The Extra Pharmacopoeia, 30th ed, p604) Levomepromazine is a phenothiazine and typical antipsychotic agent, with sedative/hypnotic, anxiolytic, antiemetic, analgesic and antipsychotic activities. Although the exact mechanism of action of levomepromazine is not fully known, upon administration, this agent appears to act as an antagonist for a variety of receptors in the central nervous system (CNS), including adrenergic, dopamine, histamine, cholinergic and serotonin (5-hydroxytryptamine; 5-HT) receptors. Blocking these receptors results in levomepromazine's pharmacologic effects. Methotrimeprazine is only found in individuals that have used or taken this drug. It is a phenothiazine with pharmacological activity similar to that of both chlorpromazine and promethazine. It has the histamine-antagonist properties of the antihistamines together with central nervous system effects resembling those of chlorpromazine. (From Martindale, The Extra Pharmacopoeia, 30th ed, p604). Methotrimeprazine's antipsychotic effect is largely due to its antagonism of dopamine receptors in the brain. In addition, its binding to 5HT2 receptors may also play a role. A phenothiazine with pharmacological activity similar to that of both CHLORPROMAZINE and PROMETHAZINE. It has the histamine-antagonist properties of the antihistamines together with CENTRAL NERVOUS SYSTEM effects resembling those of chlorpromazine. (From Martindale, The Extra Pharmacopoeia, 30th ed, p604) See also: Phenothiazine (subclass of). Drug Indication For the treatment of psychosis, particular those of schizophrenia, and manic phases of bipolar disorder. Mechanism of Action Methotrimeprazine's antipsychotic effect is largely due to its antagonism of dopamine receptors in the brain. In addition, its binding to 5HT2 receptors may also play a role. Pharmacodynamics Methotrimeprazine is a phenothiazine with pharmacological activity similar to that of both chlorpromazine and promethazine. It has the histamine-antagonist properties of the antihistamines together with central nervous system effects resembling those of chlorpromazine. (From Martindale, The Extra Pharmacopoeia, 30th ed, p604) |
Solubility Data
| Solubility (In Vitro) | DMSO: ~41.7 mg/mL (~126.9 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.43 mg/mL (4.35 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.43 mg/mL (4.35 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1.43 mg/mL (4.35 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0444 mL | 15.2221 mL | 30.4442 mL | |
| 5 mM | 0.6089 mL | 3.0444 mL | 6.0888 mL | |
| 10 mM | 0.3044 mL | 1.5222 mL | 3.0444 mL |