Physicochemical Properties
| Molecular Formula | C36H66CL4N4O2 |
| Molecular Weight | 728.75 |
| Exact Mass | 726.394 |
| CAS # | 104807-46-7 |
| PubChem CID | 107759 |
| Appearance | White to off-white solid powder |
| Boiling Point | 681.9ºC at 760mmHg |
| Flash Point | 316.7ºC |
| Vapour Pressure | 1.89E-18mmHg at 25°C |
| LogP | 11.995 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 29 |
| Heavy Atom Count | 46 |
| Complexity | 519 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | CDKGGOUDHGSFAF-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C36H62N4O2.4ClH/c1-41-35-23-13-11-21-33(35)31-39-29-19-9-7-17-27-37-25-15-5-3-4-6-16-26-38-28-18-8-10-20-30-40-32-34-22-12-14-24-36(34)42-2;;;;/h11-14,21-24,37-40H,3-10,15-20,25-32H2,1-2H3;4*1H |
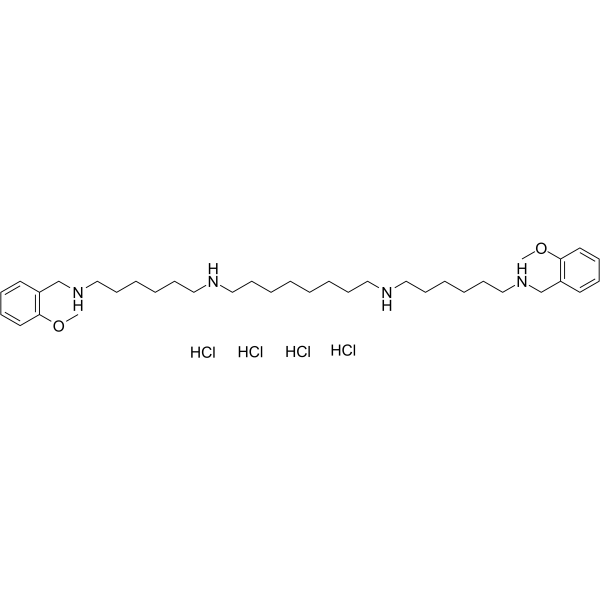
| Chemical Name | N,N'-bis[6-[(2-methoxyphenyl)methylamino]hexyl]octane-1,8-diamine;tetrahydrochloride |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | M2 muscarinic receptor[1] |
| ln Vitro | In a concentration-dependent manner, methoctramine tetrahydrochloride reduces the increases in PG production generated by acetylcholine (ACh) and arecaidine propargyl ester (APE)[1]. In the guinea-pig isolated, innervated tracheal tube preparation, methoctramine (0.01-1 μM) tetrahydrochloride facilitates contractions elicited by pre- and postganglionic nerve stimulation[2]. Methoctramine tetrahydrochloride (≥10 μM) diminishes reactions to exogenous ACh and nerve stimulation[2]. |
| ln Vivo | In anesthetized rats, bradycardia caused by methacholine and muscarine is substantially inhibited by methoctramine (300 µg/kg; iv) tetrahydrochloride[3]. |
| References |
[1]. Methoctramine, a cardioselective antagonist: muscarinic receptor mediating prostaglandin synthesis in isolated rabbit heart. Eur J Pharmacol. 1991 Jan 3;192(1):63-70. [2]. Actions of methoctramine, a muscarinic M2 receptor antagonist, on muscarinic and nicotinic cholinoceptors in guinea-pig airways in vivo and in vitro. Br J Pharmacol. 1992 Jan;105(1):107-12. [3]. Methoctramine selectively blocks cardiac muscarinic M2 receptors in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):246-9. |
| Additional Infomation | Methoctramine tetrahydrochloride is a hydrochloride obtained by combining methoctramine with four molar equivalents of hydrochloric acid. It has a role as a muscarinic antagonist. It contains a methoctramine(4+). |
Solubility Data
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3722 mL | 6.8611 mL | 13.7221 mL | |
| 5 mM | 0.2744 mL | 1.3722 mL | 2.7444 mL | |
| 10 mM | 0.1372 mL | 0.6861 mL | 1.3722 mL |