MS023 (MS-023) is a novel, potent, selective, and cell-active inhibitor of PRMTs (Protein arginine methyltransferases) with potential anticancer activity. It inhibits PRMTs with IC50 ranging from 4 to 119 nM. PRMTs play a crucial role in many biological processes and overexpression of PRMTs has been implicated in many human diseases including cancer. MS023 displayed high potency for type I PRMTs including PRMT1, -3, -4, -6, and -8 but was completely inactive against type II and type III PRMTs, protein lysine methyltransferases and DNA methyltransferases. A crystal structure of PRMT6 in complex with MS023 revealed that MS023 binds the substrate binding site. MS023 potently decreased cellular levels of histone arginine asymmetric dimethylation. It also reduced global levels of arginine asymmetric dimethylation and concurrently increased levels of arginine monomethylation and symmetric dimethylation in cells. We also developed MS094, a close analog of MS023, which was inactive in biochemical and cellular assays, as a negative control for chemical biology studies.
Physicochemical Properties
| Molecular Formula | C17H25N3O | |
| Molecular Weight | 287.4 | |
| Exact Mass | 287.199 | |
| Elemental Analysis | C, 71.04; H, 8.77; N, 14.62; O, 5.57 | |
| CAS # | 1831110-54-3 | |
| Related CAS # | MS023 dihydrochloride;1992047-64-9;MS023 trihydrochloride;2108631-19-0 | |
| PubChem CID | 92136227 | |
| Appearance | Typically exists as white to light yellow solids at room temperature | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 437.8±45.0 °C at 760 mmHg | |
| Flash Point | 218.6±28.7 °C | |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C | |
| Index of Refraction | 1.567 | |
| LogP | 2.28 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 21 | |
| Complexity | 290 | |
| Defined Atom Stereocenter Count | 0 | |
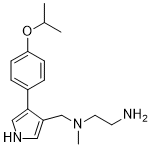
| SMILES | O(C(C)C)C1C=CC(=CC=1)C1=CNC=C1CN(C)CCN |
|
| InChi Key | FMTVWAGUJRUAKE-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C17H25N3O/c1-13(2)21-16-6-4-14(5-7-16)17-11-19-10-15(17)12-20(3)9-8-18/h4-7,10-11,13,19H,8-9,12,18H2,1-3H3 | |
| Chemical Name | N1-((4-(4-isopropoxyphenyl)-1H-pyrrol-3-yl)methyl)-N1-methylethane-1,2-diamine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PRMT1 (IC50 = 30 nM); PRMT3 (IC50 = 119 nM); PRMT4 (IC50 = 83 nM); PRMT6 (IC50 = 4 nM); PRMT8 (IC50 = 5 nM) |
| ln Vitro | In MCF7 cells, PRMT1 methyltransferase activity is inhibited by MS023 (1-1000 nM; 48 h) [1]. In HEK293 cells, MS023 (1-1000 nM; 20 h) suppresses PRMT6 methyltransferase activity [1]. |
| ln Vivo | When combined with PKC412 (100 mg/kg, ig), MS023 (160 mg/kg, ip) prevents the growth of MLL-r acute lymphoblastic leukemia (ALL) by preventing the maintenance of functional MLL-r ALL initiating cells. disperse[2]. |
| Enzyme Assay |
PRMT biochemical assays[1] A scintillation proximity assay (SPA) was used for assessing the effect of test compounds on inhibiting the methyl transfer reaction catalyzed by PRMTs as described previously.27 In brief, the tritiated S-adenosyl-L-methionine was used as the donor of methyl group. The (3H) methylated biotin labelled peptide was captured in streptavidin/scintillant-coated microplate which brings the incorporated 3H-methyl and the scintillant to close proximity resulting in light emission that is quantified by tracing the radioactivity signal (counts per minute) as measured by a TopCount NXT™ Microplate Scintillation and Luminescence Counter. When necessary, non-tritiated SAM was used to supplement the reactions. The IC50 values were determined under balanced conditions at Km concentrations of both substrate and cofactor by titration of test compounds in the reaction mixture. Cellular PRMT1 assay[1] MCF7 cells were grown in 12-well plates in DMEM supplemented with 10% FBS, penicillin (100 units mL−1) and streptomycin (100 μg mL−1). 40% confluent cells were treated with different concentrations of MS023 and compounds 4 – 6 at indicated concentrations or DMSO control for 48 h. Cells were lysed in 100 μL of total lysis buffer (20 mM Tris-HCl pH 8, 150 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 0.5% TritonX-100, 12.5 U mL−1 benzonase, complete EDTA-free protease inhibitor cocktail). After 3 min incubation at RT, SDS was added to final 1% concentration. Lysates were run on SDS-PAGE and immunoblotting was done as outlined below to determine H4R3me2a, arginine asymmetric dimethylation, arginine symmetric dimethylation and arginine monomethylation in western blot. Cellular PRMT6 assay[1] HEK293 cells were grown in 12-well plates in DMEM supplemented with 10% FBS, penicillin (100 U mL−1) and streptomycin (100 μg mL−1). 50 % confluent cells were transfected with FLAG-tagged PRMT6 or mutant V86K/D88A PRMT6 (1 μg of DNA per well) using jetPRIME® transfection reagent (Polyplus-Transfection), following manufacturer instructions. After 4 h media were removed and cells were treated with MS023 at indicated concentrations or DMSO control. After 20 h, media was removed and cells were lysed in 100 μL of total lysis buffer. |
| Cell Assay |
Western Blot Analysis[1] Cell Types: MCF7 and HEK293 cells Tested Concentrations: 1.4, 4, 12, 37, 111, 333, and 1000 nM Incubation Duration: 48 hrs (hours) for MCF7 cells; 20 hrs (hours) for HEK293 cells Experimental Results: Treatment potently and concentration -dependently decreased cellular levels of H4R3me2a (IC50=9±0.2 nM). Treatment concentration-dependently decreased the H3R2me2a mark (IC50=56±7 nM). |
| Animal Protocol |
Animal/Disease Models: NOD-scid IL2Rgnull (NSG) mice bearing primary MLL-r ALL cells[2] Doses: 160 mg/kg Route of Administration: intraperitoneal (ip)injection; PKC412 (100 mg/kg, ig), MS023 (160 mg/kg, ip ), or a combination for 4 weeks Experimental Results: Combinatorial treatment extended survival of leukemic mice relative to single treatments. In vivo treatment of MLL-r ALL-engrafted mouse model[1] For studying inducible short hairpin PRMT1 (shPRMT1), SEM cells stably expressing either doxycycline (DOX)-inducible short hairpin control (shCtrl) or PRMT1 short hairpin RNA (shRNA) (shPRMT1) were transplanted into irradiated immunodeficient NOD-scid IL2Rgnull (NSG) mice (1 × 106 cells per mouse). Each group was administered DOX treatment (10 mg/kg) orally for 3 weeks after engraftment. To assess R972/973 function in leukemogeneis, KOCL45 cells transduced with constructs expressing an FLT3 variant were sorted by using red fluorescent protein and injected into mice (1 × 105 cells per mouse), and mouse survival was monitored daily. To assess MS023 effects in vivo, we transplanted primary MLL-r ALL cells into NSG mice (0.5 × 106 cells per mouse). After engraftment, grouped mice were treated with vehicle, PKC412 (100 mg/kg, intragastrically), MS023 (160 mg/kg, intraperitoneally), or a combination for 4 weeks. After treatment, engrafted human cells were identified. Secondary transplantations of whole bone marrow (BM) cells from treated or control mice were then performed. Animal procedures were performed in accordance with federal and state government guidelines and established institutional guidelines and protocols approved by the Institutional Animal Care and Use Committee at City of Hope. |
| References |
[1]. A Potent, Selective, and Cell-Active Inhibitor of Human Type I Protein Arginine Methyltransferases. ACS Chem Biol. 2016 Mar 18;11(3):772-81. [2]. Targeting PRMT1-mediated FLT3 methylation disrupts maintenance of MLL-rearranged acute lymphoblastic leukemia. Blood. 2019 Oct 10;134(15):1257-1268. |
| Additional Infomation | MS023 is a type I PRMTs inhibitor. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 53.33 mg/mL (185.56 mM) in Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4795 mL | 17.3974 mL | 34.7947 mL | |
| 5 mM | 0.6959 mL | 3.4795 mL | 6.9589 mL | |
| 10 mM | 0.3479 mL | 1.7397 mL | 3.4795 mL |