Physicochemical Properties
| Molecular Formula | C14H13CLN4NAO9P |
| Molecular Weight | 470.69 |
| Exact Mass | 473.972 |
| CAS # | 1197030-56-0 |
| PubChem CID | 136068412 |
| Appearance | Solid powder |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 30 |
| Complexity | 632 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | VLNWYJFCKRYEJA-UHFFFAOYSA-L |
| InChi Code | InChI=1S/C14H12ClN4O8P.2Na/c1-7-13(21)9(5-20)10(6-27-28(24,25)26)14(16-7)18-17-12-4-8(19(22)23)2-3-11(12)15;;/h2-5,21H,6H2,1H3,(H2,24,25,26);;/q;2*+1/p-2 |
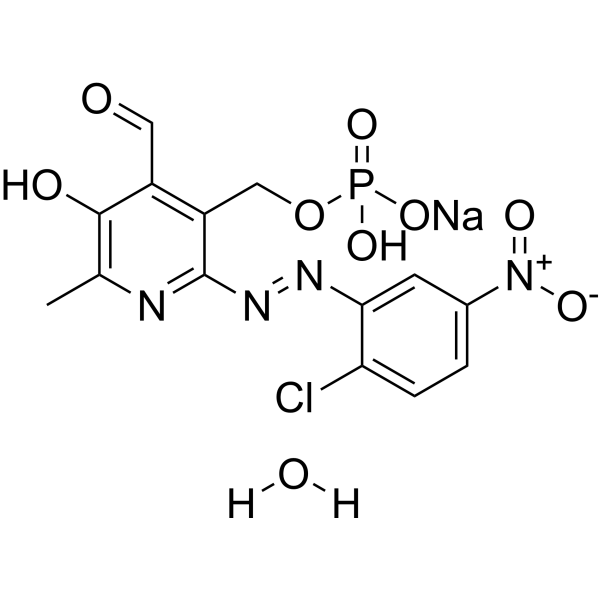
| Chemical Name | disodium;[2-[(2-chloro-5-nitrophenyl)diazenyl]-4-formyl-5-hydroxy-6-methylpyridin-3-yl]methyl phosphate |
| Synonyms | MRS 2211; 2-[(2-CHLORO-5-NITROPHENYL)AZO]-5-HYDROXY-6-METHYL-3-[(PHOSPHONOOXY)METHYL]-4-PYRIDINECARBOXALDEHYDE DISODIUM SALT; 1197030-56-0; disodium;[2-[(2-chloro-5-nitrophenyl)diazenyl]-4-formyl-5-hydroxy-6-methylpyridin-3-yl]methyl phosphate; SCHEMBL3361090; CHEMBL1909455; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | P2Y13 receptor (pIC50= 5.97) |
| ln Vitro | The 2-chloro-5-nitro analogue (MRS 2211) and 4-chloro-3-nitro analogue (MRS 2603) inhibited ADP (100 nM)-induced inositol trisphosphate (IP3) formation with pIC50 values of 5.97 and 6.18, respectively, being 45- and 74-fold more potent than PPADS. The antagonism of MRS 2211 was competitive with a pA2 value of 6.3. MRS2211 and MRS2603 inhibited phospholipase C (PLC) responses to 30 nM 2-methylthio-ADP in human P2Y1 receptor-mediated 1321N1 astrocytoma cells with IC50 values of >10 and 0.245 μM, respectively. Both analogues were inactive (IC50 > 10 μM) as antagonists of human P2Y12 receptor-mediated PLC responses in 1321N1 astrocytoma cells. Thus, MRS2211 displayed >20-fold selectivity as antagonist of the P2Y13 receptor in comparison to P2Y1 and P2Y12 receptors, while MRS2603 antagonized both P2Y1 and P2Y13 receptors [1]. |
| Enzyme Assay |
Inositol trisphosphate (IP3) assay [1] Formation of inositol trisphosphate was measured as described. Briefly, the hP2Y13-AG32 cells (200,000 cells/well) were seeded on 35-mm diameter cell culture dishes in complete medium two days before the experiment. One day before the experiment the incubation medium was changed by DMEM supplemented with 5% of FBS (v/v), antibiotics, G418, and [myo-D-2-3H]-inositol (5 μCi/ml). After 18 h this labelling medium was aspirated and the cells were incubated in Krebs–Ringer–HEPES (KRH) buffer (124 mM NaCl, 5 mM KCl, 1.25 mM MgSO4, 1.45 mM CaCl2, 1.25 mM KH2PO4, 25 mM HEPES, pH 7.4, and 8 mM D-glucose) for 2 additional hours. The cells were then pre-treated with antagonist solutions or buffer alone for 10 min at 37 °C followed by the addition of ADP for 30 s. Reactions were terminated upon aspiration of the medium and addition of 1 ml of 3% ice-cold perchloric acid solution. Inositol trisphosphate was then separated on Dowex columns as described previously [18]. Inhibition of phospholipase C (PLC) responses to 2-methylthio-ADP in human P2Y1 receptor-expressing 1321N1 cells was measured as reported, and similar methods were used for human P2Y12 receptor-mediated responses. |
| Cell Assay |
Cell culture [1] Previously constructed human astrocytoma 1321N1 cells expressing Gα16 protein and stably transfected with hP2Y13 receptors (hP2Y13-1321N1-Gα16) were used. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% of fetal bovine serum (FBS; v/v), 100 units/ml of penicillin, 100 μg/ml of streptomycin, 400 μg/ml of G418, and 500 μg/ml of zeocin. Cells were grown at 37 °C in a humidified atmosphere of 95% air and 5% CO2 in 9-cm Petri dishes. |
| References |
[1]. Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor[J]. Biochemical pharmacology, 2005, 70(2): 266-274. |
| Additional Infomation |
Researchers have synthesized a series of derivatives of the known P2 receptor antagonist PPADS (pyridoxal-5'-phosphate-6-azo-phenyl-2,4-disulfonate) and examined their ability to inhibit functional activity of the recombinant human P2Y13 nucleotide receptor expressed in 1321N1 human astrocytoma cells co-expressing G(alpha)16 protein (AG32). Analogues of PPADS modified through substitution of the phenylazo ring, including halo and nitro substitution, and 5'-alkyl phosphonate analogues were synthesized and tested. A 6-benzyl-5'-methyl phosphonate analogue was prepared to examine the effect of stable replacement of the azo linkage. The highest antagonistic potency was observed for 6-(3-nitrophenylazo) derivatives of pyridoxal-5'-phosphate. The 2-chloro-5-nitro analogue (MRS 2211) and 4-chloro-3-nitro analogue (MRS 2603) inhibited ADP (100 nM)-induced inositol trisphosphate (IP3) formation with pIC50 values of 5.97 and 6.18, respectively, being 45- and 74-fold more potent than PPADS. The antagonism of MRS 2211 was competitive with a pA2 value of 6.3. MRS2211 and MRS2603 inhibited phospholipase C (PLC) responses to 30 nM 2-methylthio-ADP in human P2Y1 receptor-mediated 1321N1 astrocytoma cells with IC50 values of >10 and 0.245 microM, respectively. Both analogues were inactive (IC50>10 microM) as antagonists of human P2Y12 receptor-mediated PLC responses in 1321N1 astrocytoma cells. Thus, MRS2211 displayed >20-fold selectivity as antagonist of the P2Y13 receptor in comparison to P2Y1 and P2Y12 receptors, while MRS2603 antagonized both P2Y1 and P2Y13 receptors. The most potent antagonists among this series were two compounds containing both chloro and the m-nitro group in the phenylazo ring: 2-chloro-5-nitro (12, MRS 2211) and 4-chloro-3-nitro (13, MRS 2603). The inhibition by these compounds of the ADP (100 nM)-induced inositol trisphosphate synthesis was already evident at high nanomolar concentrations. Compounds 12 and 13 were 45- and 74-fold, respectively, more potent at the P2Y13 receptor than the parental compound PPADS. The interaction of MRS 2211 with the P2Y13 receptor had a competitive character with a pA2 value of 6.3, calculated from the Schild plot. [1] |
Solubility Data
| Solubility (In Vitro) | H2O : 32.95 mg/mL (70.00 mM; with sonication and heat) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1245 mL | 10.6227 mL | 21.2454 mL | |
| 5 mM | 0.4249 mL | 2.1245 mL | 4.2491 mL | |
| 10 mM | 0.2125 mL | 1.0623 mL | 2.1245 mL |