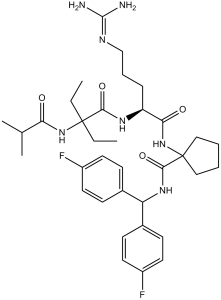
MM-102 (also known as HMTase Inhibitor IX) is a novel and potent peptidomimetic inhibitor of MLL1 (Mixed lineage leukemia 1) with anticancer activity. It inhibits MLL1 with an IC50 of 0.4 μM in a cell-free assay. MM-102 inhibited the growth of leukemia cells harboring MLL1 fusion proteins.
Physicochemical Properties
| Molecular Formula | C35H49F2N7O4 | |
| Molecular Weight | 669.8 | |
| Exact Mass | 669.381 | |
| Elemental Analysis | C, 62.76; H, 7.37; F, 5.67; N, 14.64; O, 9.55 | |
| CAS # | 1417329-24-8 | |
| Related CAS # | MM-102 TFA;1883545-52-5 | |
| PubChem CID | 54766613 | |
| Appearance | White to off-white solid powder | |
| LogP | 6.433 | |
| Hydrogen Bond Donor Count | 6 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 16 | |
| Heavy Atom Count | 48 | |
| Complexity | 1080 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | O=C(C1(NC([C@@H](NC(C(NC(C(C)C)=O)(CC)CC)=O)CCCNC(N)=N)=O)CCCC1)NC(C2=CC=C(F)C=C2)C3=CC=C(F)C=C3 |
|
| InChi Key | RZKSQRIPRKWVBU-MHZLTWQESA-N | |
| InChi Code | InChI=1S/C35H49F2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1 | |
| Chemical Name | N-[Bis(4-fluorophenyl)methyl]-1-[[(2S)-5-(diaminomethylideneamino)-2-[[2-ethyl-2-(2-methylpropanoylamino)butanoyl]amino]pentanoyl]amino]cyclopentane-1-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | MLL1-WDR5 PPI (IC50 = 2.4 nM) | |
| ln Vitro |
|
|
| ln Vivo |
MM-102 attenuates AKI after cisplatin administration in mice[2] To investigate the role of MLL1/WDR5 in cisplatin-induced AKI, mice were treated with MM102, an inhibitor of the MLL1/WDR5 complex, or vehicle 2 h before cisplatin administration (20 mg/kg, intraperitoneally injection). MM102 was then given daily for three consecutive days. Blood samples and kidney tissue were collected 72 h after cisplatin injection. Blood urea nitrogen (BUN) and serum creatinine (SCr) were used as measures of renal function. As shown in Fig. 1A, BUN levels in cisplatin group were much higher than that in control group (6.217 ± 0.374 vs. 2.420 ± 0.470 mmol/L) (***P < 0.001); MM102 treatment reduced the cisplatin-boosted BUN to 3.172 ± 0.114 mmol/L (**P < 0.01). Similarly, SCr was 68.126 ± 10.217 μmol/L in cisplatin-alone group (Fig. 1B), higher than that in the control group (10.322 ± 2.135 μmol/L) (**P < 0.01); MM102 treatment significantly reduced SCr to 20.922 ± 4.016 μmol/L (**P < 0.01); MM102 alone had little effect on either BUN or SCr. MM-102 reduces apoptosis, along with reduced p53 phosphorylation and retained E-cadherin expression in vivo[2] IF staining indicated that neutrophil gelatinase-associated lipocalin (NGAL, an early biomarker of AKI) was increased in kidneys exposed to cisplatin relative to sham-operated kidneys. Administration of MM102 dramatically reduced NGAL expression in cisplatin-injured kidneys (Fig. 2A, B). Consistently, TdT-mediated dUTP-X nick-end labeling (TUNEL) staining displayed increased number of apoptotic cells in injured kidney and MM102 largely inhibited this response (Fig. 2A, C). Moreover, increased expression of NGAL and cleavage of caspase-3 (C-cas3, a recognized marker of apoptosis) in the kidney after cisplatin administration were detected by immunoblot analysis; treatment with MM102 returned these changes to base levels. |
|
| Enzyme Assay |
Competitive Binding Assay[1] Binding affinities of all the synthesized compounds were determined using a fluorescence-polarization (FP)-based competitive binding assay; the details of this assay have been described earlier. In Vitro Histone Methyltransferase (HMT) Assay[1] The HMT assay was performed in 50 mM HEPES pH 7.8, 100 mM NaCl, 1.0 mM EDTA, and 5% glycerol at 22 °C. Each reaction contained 1.5 μCi of the co-factor,3H-S-adenosylmethionine. H3 10-residue peptide was used as the substrate at 50 μM. Compounds were added at concentrations ranging from 0.125 to 128 μM and incubated with the pre-assembled WDR5/RbBP5/ASH2L complex at a final concentration of 0.5 μM for each protein for 2–5 min. Reactions were initiated by addition of the MLL1 protein at a final concentration of 0.5 μM and allowed to proceed for 30 min before preparing scintillation counting. To count samples, reactions were spotted on separate squares of P81 filter paper (Whatman) and precipitated by submerging in freshly prepared 50 mM sodium bicarbonate buffer with pH 9.0. After washing and drying, samples were vortexed in Ultima Gold scintillation fluid and counted. As a negative control, assays were performed using 0.5 μM MLL1/WDR5/RbBP5/ASH2L complex assembled with the non-interacting mutant, WDR5D107A. |
|
| Cell Assay |
qRT-PCR Analysis of HOXA9 and MEIS-1 Genes[1] Murine MLL1-AF9 transformed bone marrow cells were obtained by transducing normal murine bone marrow cells with MLL1-AF9 oncogene according to the procedures described by Tan et al.22 MM-102 and C-MM-102 were dissolved in DMSO. The transformed cells were treated with MM-102 (25 μM, 50 μM), C-MM-102 (50 μM), and Mock (0.2% DMSO), giving a final concentration of 0.2% DMSO in all the samples. Total RNA was isolated from MLL1-AF9 transduced mouse bone marrow cells after 96 h treatment using Trizol and the RNEASY kit according to the protocol described earlier.23 The cDNA was generated using random priming with the SuperScript III kit. Real-time PCR amplifications of HoxA9, Meis1, and GAPDH genes were carried out with primers specific for each gene in the presence of SYBR dye. Relative quantification of each gene transcript was carried out as described in our previous work.10 The results were presented as relative expression to Mock treatment after normalizing to an internal loading control (e.g., GAPDH or total input RNA). Cell Growth and Apoptosis Studies of Leukemia Cell Lines[1] MV4;11, KOPN8, and K562 cells were a generous gift from Dr. Jolanta Grembecka (University of Michigan). MV4;11, KOPN8, and K562 cells were cultured in RPMI 1640 medium (ATCC) supplemented with 10% fetal bovine serum and 100 U/L penicillin-streptomycin and incubated at 37 °C under 5% CO2. Cells were seeded into 12-well plates for suspension at a density of 5 × 105 per well (1 mL) and treated with either vehicle control (DMSO, 0.2%) or MM-102 for 7 days. The medium was changed every 2 days, and compounds were resupplied. |
|
| Animal Protocol |
Animals models of AKI and treatment[2] Male C57BL/6J mice aged 6–8 weeks and weighing 20–25 g were purchased from the Jackson Laboratory. The mice were randomly divided into four groups: (1) control, (2) MM-102, (3) cisplatin, and (4) MM-102 plus cisplatin. Cisplatin was intraperitoneally injected at the dose of 20 mg/kg. MM-102 (15 mg/kg) dissolved in solvent containing 10% DMSO and 90% corn oil was administered intraperitoneally 2 h before the cisplatin injection and then given daily for three consecutive days. The dose of MM-102 was selected according to a previous report. For the control and cisplatin-alone groups, mice were injected with an equivalent amount of solvent. Mice in the control and MM-102 groups were injected with an equal volume of a normal saline solution. All the mice were euthanized 72 h after cisplatin injection. Blood samples and kidney tissues were collected for further analysis. All experimental protocols were performed according to the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals and approved by the Lifespan Animal Welfare Committee. The authorization number for the use of laboratory animals is 5074-19. |
|
| References |
[1]. High-affinity, small-molecule peptidomimetic inhibitors of MLL1/WDR5 protein-protein interaction. J Am Chem Soc. 2013, 135(2), 669-682. [2]. Histone methyltransferase MLL1 drives renal tubular cell apoptosis by p53-dependent repression of E-cadherin during cisplatin-induced acute kidney injury. Cell Death Dis . 2022 Sep 6;13(9):770. |
|
| Additional Infomation |
Mixed lineage leukemia 1 (MLL1) is a histone H3 lysine 4 (H3K4) methyltransferase, and targeting the MLL1 enzymatic activity has been proposed as a novel therapeutic strategy for the treatment of acute leukemia harboring MLL1 fusion proteins. The MLL1/WDR5 protein-protein interaction is essential for MLL1 enzymatic activity. In the present study, we designed a large number of peptidomimetics to target the MLL1/WDR5 interaction based upon -CO-ARA-NH-, the minimum binding motif derived from MLL1. Our study led to the design of high-affinity peptidomimetics, which bind to WDR5 with K(i) < 1 nM and function as potent antagonists of MLL1 activity in a fully reconstituted in vitro H3K4 methyltransferase assay. Determination of co-crystal structures of two potent peptidomimetics in complex with WDR5 establishes their structural basis for high-affinity binding to WDR5. Evaluation of one such peptidomimetic, MM-102, in bone marrow cells transduced with MLL1-AF9 fusion construct shows that the compound effectively decreases the expression of HoxA9 and Meis-1, two critical MLL1 target genes in MLL1 fusion protein mediated leukemogenesis. MM-102 also specifically inhibits cell growth and induces apoptosis in leukemia cells harboring MLL1 fusion proteins. Our study provides the first proof-of-concept for the design of small-molecule inhibitors of the WDR5/MLL1 protein-protein interaction as a novel therapeutic approach for acute leukemia harboring MLL1 fusion proteins.[1] Mixed lineage leukemia 1 (MLL1) is a histone H3 lysine 4 (H3K4) methyltransferase that interacts with WD repeat domain 5 (WDR5) to regulate cell survival, proliferation, and senescence. The role of MLL1 in the pathogenesis of acute kidney injury (AKI) is unknown. In this study, we demonstrate that MLL1, WDR5, and trimethylated H3K4 (H3K4me3) were upregulated in renal tubular cells of cisplatin-induced AKI in mice, along with increased phosphorylation of p53 and decreased expression of E-cadherin. Administration of MM102, a selective MLL1/WDR5 complex inhibitor, improved renal function and attenuated tubular injury and apoptosis, while repressing MLL1, WDR5, and H3K4me3, dephosphorylating p53 and preserving E-cadherin. In cultured mouse renal proximal tubular cells (RPTCs) exposed to cisplatin, treatment with MM102 or transfection with siRNAs for either MLL1 or WDR5 also inhibited apoptosis and p53 phosphorylation while preserving E-cadherin expression; p53 inhibition with Pifithrin-α lowered cisplatin-induced apoptosis without affecting expression of MLL1, WDR5, and H3K4me3. Interestingly, silencing of E-cadherin offset MM102's cytoprotective effects, but had no effect on p53 phosphorylation. These findings suggest that MLL1/WDR5 activates p53, which, in turn, represses E-cadherin, leading to apoptosis during cisplatin-induced AKI. Further studies showed that MM102 effectively inhibited cisplatin-triggered DNA damage response (DDR), as indicated by dephosphorylation of ataxia telangiectasia mutated (ATM) and ATM and Rad-3 related (ATR) proteins, dephosphorylation of checkpoint kinase 1 and 2 (Chk1 and Chk2); depression of γ-H2AX; and restrained cell cycle arrest, as evidenced by decreased expression of p21 and phospho-histone H3 at serine 10 in vitro and in vivo. Overall, we identify MLL1 as a novel DDR regulator that drives cisplatin-induced RPTC apoptosis and AKI by modulating the MLL1/WDR5-/ATR/ATM-Chk-p53-E-cadherin axis. Targeting the MLL1/WDR5 complex may have a therapeutic potential for the treatment of AKI.[2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4930 mL | 7.4649 mL | 14.9298 mL | |
| 5 mM | 0.2986 mL | 1.4930 mL | 2.9860 mL | |
| 10 mM | 0.1493 mL | 0.7465 mL | 1.4930 mL |