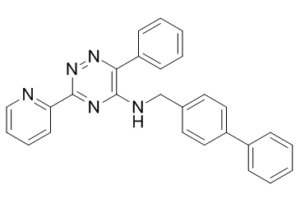
ML228 (ML-228; CID-46742353) is an activator of the Hypoxia Inducible Factor (HIF) which potently activate HIF in vitro as well as its downstream target VEGF. Hypoxia and ischemia are related to numerous public health problems affecting most major organ systems. Examples include the cardiovascular, pulmonary, renal, neurologic, and musculoskeletal systems. Furthermore, angiogenesis is required for tissue repair and regeneration. In cases of ischemia, whether due to injury or disease, enhancing blood supply is a common goal. The most significant pathway for cellular response to hypoxia is the hypoxia inducible factor (HIF) pathway. HIFs are transcription factors responsible for the activation of genes which encode proteins that mediate adaptive responses to reduced oxygen availability. The molecular probe ML228 demonstrated activity in a cell-based HIF-mediated gene reporter assay with an EC50 around 1 μM. This probe did not inhibit the proteasome, activated HIF stabilization and nuclear translocation, and induced expression of a HIF specific downstream gene (VEGF). It had no apparent toxicity below 30 μM and appeared to be an iron chelator.
Physicochemical Properties
| Molecular Formula | C₂₇H₂₁N₅ | |
| Molecular Weight | 415.49 | |
| Exact Mass | 415.179 | |
| CAS # | 1357171-62-0 | |
| Related CAS # |
|
|
| PubChem CID | 46742353 | |
| Appearance | Light yellow to yellow solid powder | |
| Density | 1.2±0.1 g/cm3 | |
| Boiling Point | 686.0±65.0 °C at 760 mmHg | |
| Flash Point | 368.7±34.3 °C | |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C | |
| Index of Refraction | 1.671 | |
| LogP | 5.05 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 32 | |
| Complexity | 536 | |
| Defined Atom Stereocenter Count | 0 | |
| InChi Key | QNRODODTMXCRKU-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C27H21N5/c1-3-9-21(10-4-1)22-16-14-20(15-17-22)19-29-27-25(23-11-5-2-6-12-23)31-32-26(30-27)24-13-7-8-18-28-24/h1-18H,19H2,(H,29,30,32) | |
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | The research community is using ML228 (CID-46742353) as a new chemotype to investigate HIF activation and its possible therapeutic applications. In addition to having a highly different structure from well-known HIF activators, ML228 is devoid of the nearly universally present acidic functional groups seen in PHD inhibitors, which could be crucial for the treatment of specific diseases [1][2]. |
| ln Vitro |
The research community is using ML228 (CID-46742353) as a new chemotype to investigate HIF activation and its possible therapeutic applications. In addition to having a highly different structure from well-known HIF activators, ML228 is devoid of the nearly universally present acidic functional groups seen in PHD inhibitors, which could be crucial for the treatment of specific diseases [1][2]. ML228 was a top active compound in three HIF-specific activity assays: a hypoxia response element (HRE)-luciferase reporter assay (EC50 = 1.23 μM), a high-content imaging assay for HIF-1α nuclear translocation (EC50 = 1.40 μM), and a real-time PCR assay for VEGF transcription induction (EC50 = 1.63 μM). It was inactive in a proteasome inhibition counterscreen assay, indicating its effects are not due to general proteasome inhibition. [1] To investigate the potential role of metal chelation in its mechanism, the effect of excess iron or zinc on ML228's activity in the HRE-luciferase assay was tested. Adding 50 μM iron to the media caused a dramatic rightward shift in the dose-response curve (EC50 shifted from 1.12 μM to 15.6 μM) and substantially decreased the magnitude of response. Adding 50 μM zinc produced a smaller rightward shift (EC50 = 5.01 μM). This suggests ML228 can chelate iron, which is consistent with some known mechanisms of HIF activation. A cell toxicity assay ruled out that the reduced activity with added metals was due to cell death. [1] ML228 was submitted to a radioligand binding assay panel of 68 GPCRs, ion channels, kinases, and transporters at 10 μM. It inhibited >75% of radioligand binding at six targets: human adenosine A3 receptor (80% inhibition), human dopamine transporter (DAT) (87%), human opiate μ receptor (85%), human potassium channel hERG (86%), human serotonin 5-HT2B receptor (92%), and rat sodium channel site 2 (105% inhibition). Moderate binding inhibition (50-75%) was observed at ten additional targets. [1] |
| ln Vivo |
Following spinal cord injury (SCI), treatment with ML228 (injection; 1 µg/kg; 7 days) can enhance the local hypoxic-ischemic environment, lessen subsequent SCI damage, and encourage neurological recovery [3]. In a rat model of spinal cord injury (SCI), treatment with ML228 (1 µg/kg) significantly improved functional recovery compared to the untreated control group, as assessed by the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale at 3 and 7 days post-operation. [3] Western blot analysis of injured spinal cord tissue showed that the expression levels of both HIF-1α and VEGF proteins were significantly higher in the ML228-treated group compared to the untreated control group at 1, 3, and 7 days after surgery. [3] Immunohistochemistry analysis of spinal cord sections confirmed that the expression and positive staining of both HIF-1α and VEGF were stronger and more prevalent in the ML228-treated group compared to the untreated control group at all time points examined (1, 3, and 7 days post-injury). [3] Hematoxylin and eosin (H&E) staining of spinal cord tissue 7 days post-operation showed that, compared to the untreated control group, the tissue structure in the ML228-treated group was more ordered and clear, with fewer necrotic areas and glial scars observed. [3] |
| Cell Assay |
A high-throughput cell-based HIF-mediated gene reporter screen was conducted using a stably transfected human U2OS osteosarcoma cell line expressing luciferase under the control of hypoxia response elements (HREs). Compounds were initially screened at 7.5 μM, and hits were further characterized with full dose-response curves. Positive control desferrioxamine (DFO, 100 μM) had an EC50 of 17.8 μM in this assay. [1] A high-content imaging assay was used to examine the accumulation and nuclear translocation of HIF-1α in U2OS cells expressing HIF-1α fused to Green Fluorescent Protein (GFP). This assay provided a secondary confirmation of HIF pathway activation. [1] A real-time PCR assay was performed to evaluate the ability of compounds to induce transcription of VEGF, a downstream target gene of the HIF pathway. [1] A commercially available cell-based proteasome inhibition assay was used as a counterscreen to eliminate compounds acting as general proteasome inhibitors. ML228 was inactive in this assay. [1] A cell toxicity assay (CellTiter-Glo®) was performed in the same U2OS cell line to rule out that the observed changes in HIF activity, particularly in the presence of added metals, were due to compound-induced cell death. No toxicity was observed under the tested conditions. [1] |
| Animal Protocol |
Animal/Disease Models: SD rats [3] Doses: 1 µg/kg Route of Administration: injection; 7-day Experimental Results: Central nervous system SCI was diminished and related symptoms were relieved. A total of 90 twelve-week-old female Sprague Dawley rats (weight 220-255 g) were used. [3] The rats were randomly divided into three groups (n=30 each): a sham group (operation without spinal cord injury), a control group (spinal cord injury without treatment), and a treatment group (spinal cord injury receiving ML228 treatment). [3] The treatment group received ML228 at a dose of 1 µg/kg. The specific route and frequency of administration are not described in detail within the provided text. [3] Spinal cord injury was induced via hemisection at the T10 level under anesthesia. Sham-operated rats underwent the same procedure but without cord transection. [3] Behavioral testing (BBB scoring) was performed prior to operation and at 1, 3, and 7 days post-operation. Animals were sacrificed at these time points for tissue collection and analysis. [3] |
| References |
[1]. Discovery of a Small Molecule Activator of the Hypoxia Inducible Factor Pathway. Probe Reports from the NIH Molecular Libraries Program. [2]. Discovery of a new molecular probe ML228: an activator of the hypoxia inducible factor (HIF) pathway. Bioorg Med Chem Lett. 2012 Jan 1;22(1):76-81. [3]. Effect of hypoxia-inducible factor-1/vascular endothelial growth factor signaling pathway on spinal cord injury in rats.Exp Ther Med. 2017 Mar;13(3):861-866. |
| Additional Infomation |
ML228 is a member of the class of 1,2,4-triazines in which the triazine ring is substituted at positions 3, 5, and 6 by pyridin-2-yl, ([biphenyl]-4-ylmethyl)amin, and methyl groups, respectively. It is an activator of the hypoxia inducible factor (HIF) pathway. It has a role as a hypoxia-inducible factor pathway activator. It is a member of biphenyls, a member of 1,2,4-triazines, a secondary amino compound and a member of pyridines. ML228 represents a novel chemotype for studying HIF activation, structurally distinct from known PHD inhibitors as it lacks the acidic functional groups (e.g., carboxylic acid) commonly found in such inhibitors. This property may be advantageous for certain applications, such as potential central nervous system targets where blood-brain barrier penetration is required. [1] Its mechanism of action is suggested to involve iron chelation, based on the significant reduction of its HRE-luciferase activity in the presence of excess iron. [1] Molecular modeling (using SurflexSim for shape-based similarity scoring) indicated a low probability that ML228 shares a similar binding mode with PHD2 as published PHD inhibitors. The shape-based similarity score was low (5.5 on a 1-10 scale), and manual docking suggested negative steric interactions in the PHD2 active site. This implies its mechanism of HIF activation might differ from classical competitive PHD inhibition. [1] ML228 is presented as a molecular probe tool compound for the research community to study HIF pathway activation and its therapeutic potential. [1] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.02 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.02 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.02 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4068 mL | 12.0340 mL | 24.0680 mL | |
| 5 mM | 0.4814 mL | 2.4068 mL | 4.8136 mL | |
| 10 mM | 0.2407 mL | 1.2034 mL | 2.4068 mL |