ML221 is a potent functional antagonist of the apelin (APJ) receptor. It originated from an HTS that gathered about 330,600 compounds using the MLSMR library. ML221 suppresses apelin-13-induced APJ activation, exhibiting IC50 values of 0.70 μM in the cAMP assay, 1.75 μM in the β-arrestin assay, and EC 80 of 10 nM in both assays. ML221 exhibits >37-fold selectivity for APJ in comparison to the closely related angiotensin II type 1 (AT1) receptor in assays conducted on cells. Apart from the κ-opioid and benzodiazepinone receptors (<50/<70%I at 10 μM), this antagonist did not exhibit any noteworthy binding activity against 29 other GPCRs.
Physicochemical Properties
| Molecular Formula | C17H11N3O6S | |
| Molecular Weight | 385.04 | |
| Exact Mass | 385.037 | |
| Elemental Analysis | C, 52.99; H, 2.88; N, 10.90; O, 24.91; S, 8.32 | |
| CAS # | 877636-42-5 | |
| Related CAS # |
|
|
| PubChem CID | 7217941 | |
| Appearance | Off-white to yellow solid powder | |
| LogP | 3.372 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 9 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 27 | |
| Complexity | 646 | |
| Defined Atom Stereocenter Count | 0 | |
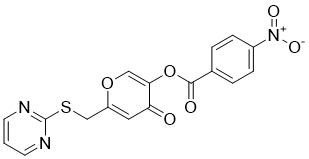
| SMILES | O=C(C1C=CC([N+](=O)[O-])=CC=1)OC1C(=O)C=C(CSC2N=CC=CN=2)OC=1 |
|
| InChi Key | UASIRTUMPRQVFY-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C17H11N3O6S/c21-14-8-13(10-27-17-18-6-1-7-19-17)25-9-15(14)26-16(22)11-2-4-12(5-3-11)20(23)24/h1-9H,10H2 | |
| Chemical Name | [4-oxo-6-(pyrimidin-2-ylsulfanylmethyl)pyran-3-yl] 4-nitrobenzoate | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | APJ ( IC50 = 1.75 μM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | ML221's antagonistic effect on apelin-13-mediated APJ activation was evaluated through two complementary APJ function assays: β-arrestin recruitment and cAMP inhibition. Increasing concentrations of ML221 antagonized a fixed concentration of Ap13 (EC80 = 10 nM) in both assays, with a calculated IC50equal to 0.70 μM in the cAMP assay, and 1.75 μM in the β-arrestin assay. | ||
| Cell Assay | The expansion of bEnd.After being incubated with ML221 (0-30 μM) for 24 hours, 3 cells are evaluated using the BrdU incorporation assay and the MTT assay. bEnd, a mouse endothelial cell line Dulbecco's modified Eagle's medium, enhanced with 10% heat-inactivated fetal bovine serum, is used to sustain three cells. For the MTT and BrdU incorporation assays, the cells are plated in 24-well culture plates at a density of 2.5 × 104/well. To perform the BrdU incorporation assay, 10 μM BrdU is added to the culture medium. The cells are fixed with 4% paraformaldehyde for 10 minutes after 2 hours. Streptavidin fluorescein isothiocyanate combined with biotinylated goat anti-mouse IgG antibodies allows for the visualization of the primary antibody. To detect nuclei, use Hoechst 33342. The number of BrdU positive cells per Hoechst positive cell is used to calculate the BrdU incorporation rate. | ||
| Animal Protocol |
|
||
| References |
[1]. Bioorg Med Chem Lett . 2012 Nov 1;22(21):6656-60. [2]. Cancer Lett . 2017 Feb 1:386:179-188. |
||
| Additional Infomation | 4-nitrobenzoic acid [4-oxo-6-[(2-pyrimidinylthio)methyl]-3-pyranyl] ester is a nitrobenzoic acid. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2 mg/mL (5.19 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5971 mL | 12.9857 mL | 25.9713 mL | |
| 5 mM | 0.5194 mL | 2.5971 mL | 5.1943 mL | |
| 10 mM | 0.2597 mL | 1.2986 mL | 2.5971 mL |