ML-18 (the S-enantiomer, the enantiomer is EMY-98), a nonpeptide analogs of PD176252, is a BRS-3 (Bombesin receptor subtype 3) antagonist with an IC50 of 4.8 μM. ML-18 and EMY-98, with IC50 values of 4.8 and >100μM, respectively, inhibited specific (125)I-BA1 (DTyr-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2)BB(6-14) binding to NCI-H1299 lung cancer cells that were stably transfected with BRS-3. On the other hand, ML-18 exhibited a lower affinity for binding to the GRPR and NMBR, with IC50 values exceeding 100μM and 16μM, respectively. With lung cancer cells loaded with FURA2-AM, ML-18 (16μM) but not its enantiomer EMY-98 inhibited 10nM BA1's capacity to elevate cytosolic Ca(2+) in a reversible manner. Lung cancer cells' ability to become tyrosine phosphorylated on EGFR and ERK in response to 100nM BA1 was inhibited by ML-18 (16μM), but not by EMY-98. ML-18 but not EMY-98 inhibited the proliferation of lung cancer cells. The findings suggest that ML-18 is a nonpeptide antagonist of BRS-3 that can be used as a model to increase potency and selectivity. A G protein coupled receptor (GPCR) for the bombesin (BB)-family of peptides is known as the bombesin receptor subtype (BRS)-3. As an orphan GPCR, BRS-3's physiological function is poorly understood because there aren't any particular agonists or antagonists. While PD176252 is a nonpeptide antagonist for the gastrin releasing peptide (GRP) R and NMBR but not BRS-3, PD168368 is a nonpeptide antagonist for the neuromedin B (NMB) receptor (R).
Physicochemical Properties
| Molecular Formula | C32H35N5O5 | |
| Molecular Weight | 569.66 | |
| Exact Mass | 569.263 | |
| Elemental Analysis | C, 67.47; H, 6.19; N, 12.29; O, 14.04 | |
| CAS # | 1422269-30-4 | |
| Related CAS # |
|
|
| PubChem CID | 91827363 | |
| Appearance | White to yellow solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 803.5±75.0 °C at 760 mmHg | |
| Flash Point | 439.7±37.1 °C | |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C | |
| Index of Refraction | 1.652 | |
| LogP | 8.03 | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 9 | |
| Heavy Atom Count | 42 | |
| Complexity | 906 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | O=C([C@]([H])(C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12)N([H])C(N([H])C1C([H])=C([H])C(=C([H])C=1[H])[N+](=O)[O-])=O)N([H])C([H])([H])C1(C2C([H])=C([H])C(=C([H])C=2[H])OC([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H] |
|
| InChi Key | JOKVJNCYOSFDGC-LJAQVGFWSA-N | |
| InChi Code | InChI=1S/C32H35N5O5/c1-42-26-15-9-23(10-16-26)32(17-5-2-6-18-32)21-34-30(38)29(19-22-20-33-28-8-4-3-7-27(22)28)36-31(39)35-24-11-13-25(14-12-24)37(40)41/h3-4,7-16,20,29,33H,2,5-6,17-19,21H2,1H3,(H,34,38)(H2,35,36,39)/t29-/m0/s1 | |
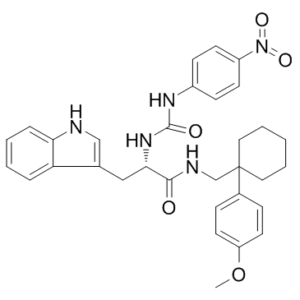
| Chemical Name | (2S)-3-(1H-indol-3-yl)-N-[[1-(4-methoxyphenyl)cyclohexyl]methyl]-2-[(4-nitrophenyl)carbamoylamino]propanamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | BRS-3 ( IC50 = 4.8 μM ) |
| ln Vitro | ML-18 blocks specific 125I-BA1 (DTyr-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2)BB6-14 binding to NCI-H1299 lung cancer cells that have been stably transfected with BRS-3 with IC50 values of 4.8 μM. ML-18 exhibits a reduced affinity for binding the GRPR and NMBR, with IC50 values exceeding 100 μM and 16 μM, respectively. ML-18 at 16 μM reversibly prevents 10 nM BA1 from increasing cytosolic Ca2+ in lung cancer cells that have been loaded with FURA2-AM. ML-18 at 16 μM prevents 100 nM BA1 from causing EGFR and ERK tyrosine phosphorylation in lung cancer cells. It prevents lung cancer cells from proliferating[1]. |
| Enzyme Assay | The cells are cultured in SIT buffer supplemented with varying concentrations of unlabelled competitor (ML-18), 0.25% bovine serum albumin, 250 μg/mL bacitracin, and 1253I-BA1 (100,000 cpm). Following a 30-minute incubation period at 37°C, free 1253I-BA1 is eliminated by three rounds of buffer washing, and the cells containing bound 1253I-BA1 are dissolved in 0.2 N NaOH and counted using a gamma counter. For every competitor without a label, the IC50 is computed[1]. |
| Cell Assay | The MTT assay is used to determine cell viability. After transfecting NCI-H727 or NCI-H1299 cells with BRS-3, ML-18 (0, 4.8, 16, 48 μM) or gefitinib is added. 15 μL of 0.1% MTT solution was added after two days. 150 μL of DMSO is added after 4 hours. The optical density at 570 nm is calculated after 16 hours[1]. |
| References |
[1]. ML-18 is a non-peptide bombesin receptor subtype-3 antagonist which inhibits lung cancer growth. Peptides. 2015 Feb;64:55-61. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (4.39 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.39 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7554 mL | 8.7772 mL | 17.5543 mL | |
| 5 mM | 0.3511 mL | 1.7554 mL | 3.5109 mL | |
| 10 mM | 0.1755 mL | 0.8777 mL | 1.7554 mL |