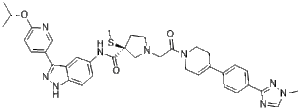
MK-8353 (also known as SCH900353; SCH-900353) is a novel, potent, selective and orally bioavailable ERK inhibitor with anticancer activity. It inhibited ERK1/2 with IC50 values of 23.0 nM and 8.8 nM, respectively, in an IMAP kinase assay, and an IC50 of 0.5 nM for nonactivated ERK2 in a MEK1-ERK2-coupled assay. In numerous preclinical cancer models, MK-8353 and SCH772984 showed comparable potency. Twenty-six patients were enrolled in the MK-8353-001 study and forty-eight patients were enrolled in the P07652 study. Diarrhoea (44%), fatigue (40%), nausea (32%), and rash (28%) were some of the negative effects. In the dose cohorts of 400 mg and 800 mg, dose-limiting toxicity was noted. Biological activity in preclinical data was found to be correlated with sufficient exposure to MK-8353. In the MK-8353-001 study, three out of fifteen patients with BRAFV600-mutant melanomas who were evaluable for treatment response showed partial response.
Physicochemical Properties
| Molecular Formula | C37H41N9O3S | |
| Molecular Weight | 691.8449 | |
| Exact Mass | 691.31 | |
| Elemental Analysis | C, 64.23; H, 5.97; N, 18.22; O, 6.94; S, 4.63 | |
| CAS # | 1184173-73-6 | |
| Related CAS # | 1184173-73-6;1951448-73-9 (HCl);1951448-74-0 (HCl hydrate);1951428-60-6 (hydrate); 1951428-61-7 (x HCl); | |
| PubChem CID | 58282870 | |
| Appearance | Off-white to light brown solid powder | |
| LogP | 4.3 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 9 | |
| Rotatable Bond Count | 10 | |
| Heavy Atom Count | 50 | |
| Complexity | 1220 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | CC(C)OC1=NC=C(C=C1)C2=NNC3=C2C=C(C=C3)NC(=O)[C@@]4(CCN(C4)CC(=O)N5CCC(=CC5)C6=CC=C(C=C6)C7=NN(C=N7)C)SC |
|
| InChi Key | KPQQGHGDBBJGFA-QNGWXLTQSA-N | |
| InChi Code | InChI=1S/C37H41N9O3S/c1-24(2)49-32-12-9-28(20-38-32)34-30-19-29(10-11-31(30)41-42-34)40-36(48)37(50-4)15-18-45(22-37)21-33(47)46-16-13-26(14-17-46)25-5-7-27(8-6-25)35-39-23-44(3)43-35/h5-13,19-20,23-24H,14-18,21-22H2,1-4H3,(H,40,48)(H,41,42)/t37-/m0/s1 | |
| Chemical Name | (3S)-3-methylsulfanyl-1-[2-[4-[4-(1-methyl-1,2,4-triazol-3-yl)phenyl]-3,6-dihydro-2H-pyridin-1-yl]-2-oxoethyl]-N-[3-(6-propan-2-yloxypyridin-3-yl)-1H-indazol-5-yl]pyrrolidine-3-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | ERK2 (IC50 = 8.8 nM); ERK1 (IC50 = 23 nM) |
| ln Vitro | MK-8353 is a potent and selective inhibitor of both active and dormant ERK1 and ERK2 kinases (IC50 = 20 and 7 nM, respectively). MK-8353 inhibits CYP 3A4 (pre-incubation) in vitro and exhibits inhibition of CYP 3A4 and 2C8 (IC50 = 1.7 & 3.5 μM), which can cause drug-drug interactions when co-administered with drugs that are primarily metabolized by CYP 2C8 or 3A4. It does not inhibit human CYPs 1A2, 2C9, 2C19, or 2D6. At 0.6 μM, MK-8353 inhibits hERG current by 16%. In A2058, HT-29, and Colo-205 cells, the IC50 values for inhibiting cell prolifertion are 371, 51, and 23 nM, respectively. MK-8353 also stops MEK from phosphorylating ERK, in addition to inhibiting ERK's kinase activity[1]. MK-8353 exhibits kinase selectivity over a 227-human kinase panel; only 3 kinases (CLK2, FLT4, and Aurora B) are inhibited >50% at the 1.0 μM concentration.No other kinase in the panel is inhibited by more than 35% at the 0.1 μM concentration. |
| ln Vivo | In male CD1 mice, Sprague Dawley (SD) rats, guinea pigs, beagle dogs, and cynomologus monkeys, the in vivo pharmacokinetics and metabolism of MK-8353 are examined. A half-life range of 1.3-2.8 hours and a mean residence time range of 1.5-4.0 hours are displayed by MK-8353 in all species, with the exception of monkeys, following IV administration. Dogs, mice, and rats all have a respectable oral bioavailability of between 23 and 80 percent, but monkeys have a low oral bioavailability of only 2%. Given the high (135 nm/sec) intestinal permeability and absorption seen in Caco-2 cells, it is likely that these parameters are also high in human beings. Rats have a steady-state volume of distribution that is 0.1 L/kg, whereas it ranges from 0.9 to 3.3 L/kg in mice, dogs, and monkeys. In numerous BRAF-mutant models, MK-8353 exhibits anti-tumor efficacy[1]. |
| Cell Assay | A2058 cells (1 × 106 cells per 10-cm dish) were given progressively higher doses of MK-8353 (nM) for 24 hours. Immunoblot analysis was conducted on whole cell lysates. |
| Animal Protocol |
Female athymic nude mice (inoculated with Colo-205 cancer cells) or SCID mice (inoculated with SK-MEL-28 melanoma cells) 30, 45 and 60 mg/kg p.o. |
| References |
[1]. ACS Med Chem Lett . 2018 Jun 14;9(7):761-767. [2]. JCI Insight . 2018 Feb 22;3(4):e92352. |
| Additional Infomation |
MK-8353 is a member of the class of indazoles that is 1H-indazole substituted by a 6-(propan-2-yloxy)pyridin-3-yl group at position 3 and by a {[(3S)-3-(methylsulfanyl)-1-(2-{4-[4-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl]-3,6-dihydropyridin-1(2H)-yl}-2-oxoethyl)pyrrolidin-3-yl]carbonyl}amino group at position 5. It is a potent and selective inhibitor of ERK1 and ERK2 in vitro (IC50 values of 23.0 nM and 8.8 nM, respectively). The drug is being developed by Merck Sharp & Dohme and is currently in clinical development for the treatment of advanced/metastatic solid tumors. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of indazoles, a member of triazoles, a dihydropyridine, a member of pyridines, an aromatic ether, a secondary carboxamide, a pyrrolidinecarboxamide, a N-alkylpyrrolidine, a methyl sulfide and a tertiary carboxamide. ERK Inhibitor MK-8353 is an orally available inhibitor of extracellular signal-regulated kinase (ERK), with potential antineoplastic activity. Upon oral administration, MK-8353 inhibits both ERK phosphorylation and activation of ERK-mediated signal transduction pathways; thereby, preventing ERK-dependent tumor cell proliferation and survival. The mitogen-activated protein kinase (MAPK)/ERK pathway is often upregulated in a variety of tumor cell types and plays a role in tumor cell proliferation, differentiation and survival. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.01 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.01 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4454 mL | 7.2271 mL | 14.4542 mL | |
| 5 mM | 0.2891 mL | 1.4454 mL | 2.8908 mL | |
| 10 mM | 0.1445 mL | 0.7227 mL | 1.4454 mL |